Therapeutic Effect of Benidipine on Medication-Related Osteonecrosis of the Jaw
Abstract
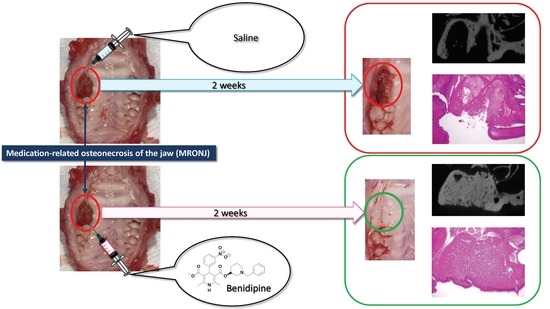
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. MRONJ-like Rat Model
4.3. Application of BD
4.4. μCT Examination and Morphometry
4.5. Histology and Histomorphometry
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Qi, W.X.; Tang, L.N.; He, A.N.; Yao, Y.; Shen, Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. 2014, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Miles, D.; Robert, N.; Diéras, V.; Glaspy, J.; Smith, I.; Thomssen, C.; Biganzoli, L.; Taran, T.; Conte, P. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010, 122, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Rückschloß, T.; Müller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Craniomaxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Melea, P.I.; Melakopoulos, I.; Kastritis, E.; Tesseromatis, C.; Margaritis, V.; Dimopoulos, M.A.; Terpos, E. Conservative treatment of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma patients. Int. J. Dent. 2014, 2014, 427273. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, R.; Panzarella, V.; Maniscalco, L.; Bedogni, A.; Licata, M.E.; Albanese, A.; Toia, F.; Cumbo, E.M.G.; Mazzola, G.; Di Fede, O.; et al. Conservative Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Er,Cr:YSGG Laser and Platelet-Rich Plasma: A Longitudinal Study. BioMed Res. Int. 2018, 2018, 3982540. [Google Scholar] [CrossRef]
- Stanton, D.C.; Balasanian, E. Outcome of surgical management of bisphosphonate-related osteonecrosis of the jaws: Review of 33 surgical cases. J. Oral Maxillofac. Surg. 2009, 67, 943–950. [Google Scholar] [CrossRef]
- Kakehashi, H.; Ando, T.; Minamizato, T.; Nakatani, Y.; Kawasaki, T.; Ikeda, H.; Kuroshima, S.; Kawakami, A.; Asahina, I. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: Preliminary findings. Int. J. Oral Maxillofac. Surg. 2015, 44, 1558–1564. [Google Scholar] [CrossRef]
- Fournier, P.; Boissier, S.; Filleur, S.; Guglielmi, J.; Cabon, F.; Colombel, M.; Clézardin, P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002, 62, 6538–6544. [Google Scholar]
- Allen, M.R.; Burr, D.B. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J. Oral Maxillofac. Surg. 2008, 66, 987–994. [Google Scholar] [CrossRef]
- Lesclous, P.; Abi Najm, S.; Carrel, J.P.; Baroukh, B.; Lombardi, T.; Willi, J.P.; Rizzoli, R.; Saffar, J.L.; Samson, J. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone 2009, 45, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Matsuzaki, M.; Matsuoka, H.; Shimamoto, K.; Shimada, K.; Rakugi, H.; Umemoto, S.; Kamiya, A.; Suzuki, N.; Kumagai, H.; et al. The combination therapy of hypertension to prevent cardiovascular events (COPE) trial: Rationale and design. Hypertens. Res. 2005, 28, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Cheng, X.W.; Jin, Z.; Obata, K.; Nagata, K.; Hirashiki, A.; Sasaki, T.; Noda, A.; Takeshita, K.; Izawa, H.; et al. Ca2+ channel blocker benidipine promotes coronary angiogenesis and reduces both left-ventricular diastolic stiffness and mortality in hypertensive rats. J. Hypertens. 2010, 28, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Ogihara, T.; Umemoto, S.; Rakugi, H.; Matsuoka, H.; Shimada, K.; Abe, K.; Suzuki, N.; Eto, T.; Higaki, J.; et al. Prevention of cardiovascular events with calcium channel blocker-based combination therapies in patients with hypertension: A randomized controlled trial. J. Hypertens. 2011, 29, 1649–1659. [Google Scholar] [CrossRef]
- Imai, M.; Ayukawa, Y.; Yasunami, N.; Furuhashi, A.; Takemura, Y.; Adachi, N.; Hu, J.; Zhou, X.; Moriyama, Y.; Atsuta, I.; et al. Effect of a Single Injection of Benidipine-Impregnated Biodegradable Microcarriers on Bone and Gingival Healing at the Tooth Extraction Socket. Adv. Wound Care 2019, 8, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, Y.; Sugimoto, S. Effects of various antihypertensive drugs on the function of osteoblast. Biol. Pharm. Bull. 2001, 24, 628–633. [Google Scholar] [CrossRef]
- Nishiya, Y.; Kosaka, N.; Uchii, M.; Sugimoto, S. A potent 1,4-dihydropyridine L-type calcium channel blocker, benidipine, promotes osteoblast differentiation. Calcif. Tissue Int. 2002, 70, 30–39. [Google Scholar] [CrossRef]
- Wang, B.; Bi, M.; Zhu, Z.; Wu, L.; Wang, J. Effects of the antihypertensive drug benidipine on osteoblast function in vitro. Exp. Ther. Med. 2014, 7, 649–653. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakagami, H.; Yasumasa, N.; Mariana, O.K.; Kyutoku, M.; Koriyama, H.; Nakagami, F.; Shimamura, M.; Rakugi, H.; Morishita, R. Cilnidipine, but not amlodipine, ameliorates osteoporosis in ovariectomized hypertensive rats through inhibition of the N-type calcium channel. Hypertens. Res. 2012, 35, 77–81. [Google Scholar] [CrossRef]
- Adachi, N.; Ayukawa, Y.; Yasunami, N.; Furuhashi, A.; Imai, M.; Sanda, K.; Atsuta, I.; Koyano, K. Preventive effect of fluvastatin on the development of medication-related osteonecrosis of the jaw. Sci. Rep. 2020, 10, 5620. [Google Scholar] [CrossRef] [PubMed]
- Sugano, N.; Wakino, S.; Kanda, T.; Tatematsu, S.; Homma, K.; Yoshioka, K.; Hasegawa, K.; Hara, Y.; Suetsugu, Y.; Yoshizawa, T.; et al. T-type calcium channel blockade as a therapeutic strategy against renal injury in rats with subtotal nephrectomy. Kidney Int. 2008, 73, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Yoshioka, K.; Nakase, T.; Itoh, K. Stimulation of ectopic bone formation in response to BMP-2 by Rho kinase inhibitor: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.P.; Liao, J.C.; Zhao, C.; Cai, D.Z. Effects of the 1,4-dihydropyridine L-type calcium channel blocker benidipine on bone marrow stromal cells. Cell Tissue Res. 2015, 361, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Meng, Q.; Zhang, P.; Yan, P.; Zhang, Z.; Zhang, H.; Pan, J.; Zhai, Y.; Liu, Y.; et al. Chronic Calcium Channel Inhibitor Verapamil Antagonizes TNF-α-Mediated Inflammatory Reaction and Protects Against Inflammatory Arthritis in Mice. Inflammation 2016, 39, 1624–1634. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. In Macrophages; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1784, pp. 119–126. [Google Scholar] [CrossRef]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.I.; Kühl, A.A.; Erben, U.; May, C.; Schulzke, J.D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, R.; Du, J.; Fu, Y.; Li, S.; Zhang, P.; Liu, L.; Jiang, H. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J. 2019, 33, 5208–5219. [Google Scholar] [CrossRef]
- Ono, M.; Ohno, N.; Hasegawa, K.; Tanaka, S.; Komiya, M.; Matsumoto, H.; Fujii, A.; Akimoto, Y. Incidence of gingival overgrowth caused by calcium channel blockers. Jpn. Soc. Oral. Ther. Pharmacol. 2008, 27, 79–85. [Google Scholar]
- Ichida, M.; Yui, Y.; Yoshioka, K.; Tanaka, T.; Wakamatsu, T.; Yoshikawa, H.; Itoh, K. Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Lett. 2011, 585, 4018–4024. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kobayashi, S.; Dalrymple, P.D.; Wood, S.G.; Chasseaud, L.F. Absorption, metabolism and excretion after oral administration of a new Ca antagonist, 14C-benidipine hydrochloride to man. Xenobiotica 1997, 27, 597–608. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Yamato, M.; Okano, T.; Ando, T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016, 42, 400–410. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaka, K.; Imai, M.; Sanda, K.; Yasunami, N.; Furuhashi, A.; Atsuta, I.; Wada, H.; Ayukawa, Y. Therapeutic Effect of Benidipine on Medication-Related Osteonecrosis of the Jaw. Pharmaceuticals 2022, 15, 1020. https://doi.org/10.3390/ph15081020
Matsunaka K, Imai M, Sanda K, Yasunami N, Furuhashi A, Atsuta I, Wada H, Ayukawa Y. Therapeutic Effect of Benidipine on Medication-Related Osteonecrosis of the Jaw. Pharmaceuticals. 2022; 15(8):1020. https://doi.org/10.3390/ph15081020
Chicago/Turabian StyleMatsunaka, Ken, Mikio Imai, Koma Sanda, Noriyuki Yasunami, Akihiro Furuhashi, Ikiru Atsuta, Hiroko Wada, and Yasunori Ayukawa. 2022. "Therapeutic Effect of Benidipine on Medication-Related Osteonecrosis of the Jaw" Pharmaceuticals 15, no. 8: 1020. https://doi.org/10.3390/ph15081020
APA StyleMatsunaka, K., Imai, M., Sanda, K., Yasunami, N., Furuhashi, A., Atsuta, I., Wada, H., & Ayukawa, Y. (2022). Therapeutic Effect of Benidipine on Medication-Related Osteonecrosis of the Jaw. Pharmaceuticals, 15(8), 1020. https://doi.org/10.3390/ph15081020