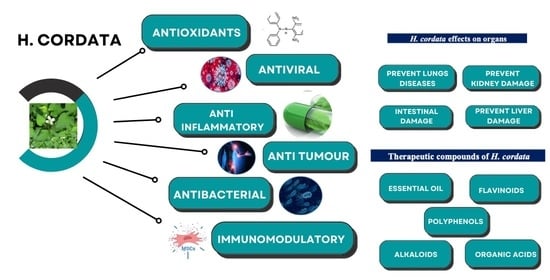
Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review
Abstract
:1. Introduction
2. Chemical Components of H. cordata
| Class | Compound | Chemical Structure | Therapeutic Properties | References |
|---|---|---|---|---|
| Essential Oil (Oil) | Terpenoids |  | Anti-bacterial activities, anti-viral activities, and anti-inflammatory activities. | [12,33,34] |
| Hydrocarbons |  1-Hexadecene | Anti-bacterial activities, anti-viral activities, and anti-inflammatory activities. | [12,30,33] | |
| Esters |  | Anti-bacterial activities and anti--inflammatory activities. | [12,33] | |
| Alcohols |  | Anti-viral activities and anti-inflammatory activities. | [12,30,33] | |
| Flavonoids | Quercetin |  | Inflammatory mediator, anti-viral | [9,35] |
| Quercitrin |  | Anti-inflamatory | [36] | |
| Hyperin |  | Anti-viral, anti-inflamatory | [9,37] | |
| Rutin |  | Inhibition of cholestasis | [9,38] | |
| Afzelin |  | Lower inflamatory cytokines | [28,39] | |
| Kaempferol |  | Anti-inflamatory | [9] | |
| Isoquercitrin |  | Anti-inflamatory | [9] | |
| Alkaloids (Alkaloids) | Aristolactam A |  | Anti-tumor activities | [9,17] |
| Aristolactam B |  | Anti-inflamatory | [9,23] | |
| Piperolactam A |  | Anti-bacterial, anti-pyretic, detoxicant, anti-ulcer | [9,17] | |
| Lysicamine |  | Anti-parasitic, anti-bacterial | [9,40] | |
| Norcepharadione B |  | Anti-inflamatory, anti-pyretic | [9,17] | |
| 3,4-Dimethoxy-N-methyl aristolactam |  | Anti-inflamatory | [9] | |
| cis-N-(4-Hydroxystyryl) benzamide |  | Inhibitor of platelet aggregation | [9,41]. | |
| 3,5-Didecanoyl-4-nonyl-1, 4-dihydropyridine |  | Anti-inflamatory | [9,21] | |
| 7-chloro-6- demethylcepharadione B |  | Anti-oxidant | [9] | |
| trans-N-(4-Hydroxystyryl) benzamide |  | Inhibitor of platelet aggregation | [41] | |
| Cepharadione B |  | Anti-oxidant | [9] | |
| Splendidine |  | Inhibitor of platelet aggregation | [9,42] | |
| Organic Acid |  | Anti-bacterial, anti-fungal activities | [43] | |
| Polyphenols | Houttuynamide A |  | Anti-inflamatory | [9] |
| Houttuynaside A |  | Anti-inflamatory | [9] | |
| Chlorogenic acid |  | Anti-hypertensive | [9] | |
| Vanillin |  | Anti-oxidant | [9] | |
3. Anti-Inflammatory Effects and Immunomodulatory Activity of H. cordata
3.1. In Vitro Anti-Inflamatory Effect of H. cordata
3.2. In Vivo Anti-Inflamatory Effect of H. cordata
| Extracts | Doses | Standard Drug | Inflammagen Used | Model Used | Time Period | Minimal Active Concentration | Most Potent Biomolecule | Reference |
|---|---|---|---|---|---|---|---|---|
| Aqueous | 0.5–3 g/kg | None | DNA-BSA | ICR mouse | 2 h | 0.5 g/kg | Not mentioned | [55] |
| Aqueous | 1, 10, 20 µg/mL | None | DNA-BSA | RBL-2H3 | 30 min | 1 µg/mL | Not mentioned | [55] |
| Aqueous | 1, 2 g/mL | None | Acetaminophen | BALB/cA mice | 4 weeks | 2 g/L | Not mentioned | [29] |
| Ethanol (100%) | 0.1, 0.2, 1% | None | LTA (1 µg/mL) | RT-7 | 24 h | 0.5% | Not mentioned | [19] |
| Ethanol(80%) | 400, 600, 100 mg/kg | None | Oxaliplatin | Male Sprague Dawley rat | 15 days | 1000 mg/kg/day | Not mentioned | [56] |
| Ethanol | 0.05, 0.1, 0.2 mg/mL | None | PMA + Ca2+ ionophore | HMC-1 | 5 h | 0.2 mg/mL | Not mentioned | [57] |
| Essential oil | 0.01, 0.1, 1, 10, 100 µg/mL | NS-398 | LPS (1 µg/mL) | Mouse peritoneal macrophange | 24 h | 100 µg/mL | Not mentioned | [58] |
| Essential oil (sodium houttuyfonate and two undecanone) | 0.1,1,10,20 µg/mL | None | LPS (1 µg/mL) | RAW 264.7 | 24 h | 1 µg/mL | Sodium houttuyfonate | [29] |
| Essential oil (sodium houttuyfonate and 2 undecanone) | 100, 200, 400 mg/kg | Aspirin | Xylene | Mouse | 30 min | 200 mg/kg | Sodium houttuyfonate | [29] |
| Polysaccrides | 40, 80, 160 mg/kg | Dexamethasone | LPS | BALB/cA mice | 24 h | 40 mg/kg | Not mentioned | [59] |
| Essential oil | 20, 40 mg/kg | Dexamethasone | Xylene, Formaldehyde | Mouse | 7 days | 20 mg/kg | 2-undecane, n-Decanoic acid, Hexadecanoic acid-methyl ester, | [33] |
4. Effect of H. cordata on Different Organs
5. Anti-Oxidant Effects of Various Extracts of H. cordata
6. Anti-Tumor Activity of H. cordata
7. Effect of H. cordata on Viruses
| Name of Extract | Name of Virus | Reference |
|---|---|---|
| Quercetin 7-rhamnoside (Q7R) | porcine epidemic diarrhea virus | [82] |
| Hyperoside, rutin, quercitrin | H1N1 | [37] |
| Hot water extract | Herpes simplex virus | [84,88] |
| H. cordata water extract | dengue virus serotype 2 strains 16,681 | [84] |
| H. cordata water extract | coronavirus 2 (SARSCOV2) | [84] |
| H. cordata polysaccharides | Murine Norovirus-1 | [89] |
| Houttuynoid A | Herpes Simplex Virus type-1 | [88] |
| Quercetin, quercetrin and cinanserin | Dengue fever virus, Corona virus | [90] |
| H. cordata extract | Enteric Virus | [91] |
| H. cordata essential oil | Avian infectious bronchitis | [92] |
8. Anti-Bacterial Effect of H. cordata
9. Toxicity of H. cordata
10. Conclusions
11. Future Aspects
- Mechanisms of H. cordata, such as interaction with cells, the cell membrane, and various drugs, will be of great importance.
- Studies on its relation to the blood–brain barrier, lipophilicity, cAMP signaling, and skin permeability with pharmaceutical effects will be of utmost use. However, its possible side-effects and toxic effects should be studied carefully.
- More data are required to study the pharmacological and toxicological activity of H. cordata.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drašar, P.; Moravcova, J. Recent Advances in Analysis of Chinese Medical Plants and Traditional Medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Drasar, P.B.; Khripach, V.A. Growing Importance of Natural Products Research. Molecules 2020, 25, 6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the Liver-Gut System: Pharmacological Bases Explaining Its Therapeutic Effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef] [PubMed]
- Talman, A.M.; Clain, J.; Duval, R.; Ménard, R.; Ariey, F. Artemisinin Bioactivity and Resistance in Malaria Parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal Plants in Therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Chopra, R.N. Glossary of Indian Medicinal Plants; CSIR: New Delhi, India, 1956. [Google Scholar]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia Cordata Thunb: An Ethnopharmacological Review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef]
- Kumar, P.S.; Arivuchelvan, A.; Jagadeeswaran, A.; Punniamurthy, N.; Selvaraj, P.; Jagatheesan, P.N.R.; Mekala, P. Formulation of Enrofloxacin SLNs and Its Pharmacokinetics in Emu (Dromaius novaehollandiae) Birds. Appl. Nanosci. 2015, 5, 661–671. [Google Scholar] [CrossRef]
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A Review of Phytochemistry and Pharmacology and Quality Control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Li, G.Z.; Chai, O.H.; Lee, M.S.; Han, E.H.; Kim, H.T.; Song, C.H. Inhibitory Effects of Houttuynia Cordata Water Extracts on Anaphylactic Reaction and Mast Cell Activation. Biol. Pharm. Bull. 2005, 28, 1864–1868. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, Y.H.; Thien, P.F.; Chang, S.C.; Chen, Y.C.; Lo, S.S.; Yang, S.H.; Chen, J.L. Identifying Core Herbal Treatments for Children with Asthma: Implication from a Chinese Herbal Medicine Database in Taiwan. Evid.-Based Complement. Altern. Med. 2013, 2013, 125943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.M.; Liang, Y.Z.; Yi, L.Z.; Wu, X.J. Anti-Inflammatory Effect of Houttuynia Cordata Injection. J. Ethnopharmacol. 2006, 104, 245–249. [Google Scholar] [CrossRef]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-Inflammatory Activity of Herbal Medicines: Inhibition of Nitric Oxide Production and Tumor Necrosis Factor-α Secretion in an Activated Macrophage-like Cell Line. Am. J. Chin. Med. 2005, 33, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chang, J.S.; Chen, C.C.; Ng, L.T.; Lin, C.C. Anti-Herpes Simplex Virus Activity of Bidens Pilosa and Houttuynia Cordata. Am. J. Chin. Med. 2003, 31, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Yen, F.L.; Liao, C.W.; Lin, C.C. Protective Effect of Houttuynia Cordata Extract on Bleomycin-Induced Pulmonary Fibrosis in Rats. Am. J. Chin. Med. 2007, 35, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chiang, L.C.; Chen, C.C.; Liu, L.T.; Wang, K.C.; Lin, C.C. Atileukemic Activity of Bidenspilosa l. Var. Minor (Blume) Sherff and Houttuynia Cordata Thunb. Am. J. Chin. Med. 2001, 29, 303–312. [Google Scholar] [CrossRef]
- Kim, S.K.; Ryu, S.Y.; No, J.; Choi, S.U.; Kim, Y.S. Cytotoxic Alkaloids from Houttuynia Cordata. Arch. Pharm. Res. 2001, 24, 518–521. [Google Scholar] [CrossRef]
- Zhuang, T.; Li, F.; Huang, L.R.; Liang, J.Y.; Qu, W. Secondary Metabolites from the Plants of the Family Saururaceae and Their Biological Properties. Chem. Biodivers. 2015, 12, 194–220. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-Bacterial and Anti-Inflammatory Effects of Ethanol Extract from Houttuynia Cordata Poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef]
- Li, J.; Rehman, M.U.; Zhang, H.; Iqbal, M.K.; Mehmood, K.; Huang, S.; Nabi, F.; Sciences, A. Antibacterial Effect of the Water Extract of Houttuynia Cordata Water Extract Against Multi-Drug Resistant. Southeast Asian J. Trop. Med. Public Health 2017, 48, 1260–1266. [Google Scholar]
- Bauer, R.; Pröbstle, A.; Lotter, H.; Wagner-Redecker, W.; Matthiesen, U. Cyclooxygenase Inhibitory Constituents from Houttuynia Cordata. Phytomedicine 1996, 2, 305–308. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Wang, Z.; Liu, W.; Sang, Z.; Li, Y.; Huang, H. Bioactive Alkaloids from the Aerial Parts of Houttuynia Cordata. J. Ethnopharmacol. 2017, 195, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Probstle, A.; Bauer, R. Aristolactams and a 4,5-Dioxoaporphine Derivative from Houttuynia Cordata. Planta Med. 1992, 58, 568–569. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Rapid Screening and Identification of Antioxidants in Aqueous Extracts of Houttuynia Cordata Using LC-ESI-MS Coupled with DPPH Assay. Food Chem. 2009, 117, 750–756. [Google Scholar] [CrossRef]
- Chou, S.C.; Su, C.R.; Ku, Y.C.; Wu, T.S. The Constituents and Their Bioactivities of Houttuynia Cordata. Chem. Pharm. Bull. 2009, 57, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, H.; Wang, W.; Yu, L.; Chen, G. Determination of Flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by Capillary Electrophoresis with Electrochemical Detection. Talanta 2006, 68, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in Chemical Composition and Antibacterial Activities of Essential Oils from Two Species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef]
- Ahn, J.; Chae, H.S.; Chin, Y.W.; Kim, J. Alkaloids from Aerial Parts of Houttuynia Cordata and Their Anti-Inflammatory Activity. Bioorganic Med. Chem. Lett. 2017, 27, 2807–2811. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Shi, C.; Fang, J. A Comparative Study of Sodium Houttuyfonate and 2-Undecanone for Their in Vitro and in Vivo Anti-Inflammatory Activities and Stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical Composition and Determination of the Antibacterial Activity of Essential Oils in Liquid and Vapor Phases Extracted from Two Different Southeast Asian Herbs-Houttuynia cordata (Saururaceae) and Persicaria odorata (Polygonaceae). Molecules 2020, 25, 2432. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A.; et al. Chemical Composition and Allelopathic, Antibacterial, Antifungal, and Antiacetylcholinesterase Activity of Fish-Mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers. 2017, 14, 10. [Google Scholar] [CrossRef]
- Chen, S.D.; Gao, H.; Zhu, Q.C.; Wang, Y.Q.; Li, T.; Mu, Z.Q.; Wu, H.L.; Peng, T.; Yao, X.S. Houttuynoids A-E, Anti-Herpes Simplex Virus Active Flavonoids with Novel Skeletons from Houttuynia Cordata. Org. Lett. 2012, 14, 1772–1775. [Google Scholar] [CrossRef]
- Li, W.; Fan, T.; Zhang, Y.; Fan, T.; Zhou, P.; Niu, X.; He, L. Houttuynia Cordata Thunb. Volatile Oil Exhibited Anti-Inflammatory Effects in Vivo and Inhibited Nitric Oxide and Tumor Necrosis Factor-α Production in LPS-Stimulated Mouse Peritoneal Macrophages in Vitro. Phyther. Res. 2013, 27, 1629–1639. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, R.; Goyal, A. Chemistry of Terpenoids. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 272–278. [Google Scholar]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the Aerial Parts of Houttuynia Cordata Attenuate Lung Inflammation in Mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prasad, S.; Hemalatha, S. A Current Update on the Phytopharmacological Aspects of Houttuynia Cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.-J.; Lu, Y.; Zhang, Y.-Y.; Zhu, H.-Y.; Tu, P.; Li, H.; Chen, D.-F. Flavonoids from Houttuynia Cordata Attenuate H1N1-Induced Acute Lung Injury in Mice via Inhibition of Influenza Virus and Toll-like Receptor Signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, X.; Yu, L.; Zhu, J.; Ma, R.; Yang, X. Chemical Composition and Hepatoprotective Effects of Polyphenol-Rich Extract from Houttuynia Cordata Tea. J. Agric. Food Chem. 2012, 60, 4641–4648. [Google Scholar] [CrossRef]
- Lee, S.-B.; Kang, J.W.; Kim, S.J.; Ahn, J.; Kim, J.; Lee, S.M. Afzelin Ameliorates D-Galactosamine and Lipopolysaccharide-Induced Fulminant Hepatic Failure by Modulating Mitochondrial Quality Control and Dynamics. Br. J. Pharmacol. 2017, 174, 195–209. [Google Scholar] [CrossRef]
- Ge, Y.-C.; Wang, K.-W. New Analogues of Aporphine Alkaloids. Mini-Rev. Med. Chem. 2018, 18, 1590–1602. [Google Scholar] [CrossRef]
- Iwahori, A.R. NII-Electronic Library Service. Chem. Pharm. Bull. 1970, 2091. [Google Scholar]
- Qu, W.; Wu, F.H.; Li, J.; Liang, J.Y. Alkaloids from Houttuynia Cordata and Their Antiplatelet Aggregation Activities. Chin. J. Nat. Med. 2011, 9, 425–428. [Google Scholar] [CrossRef]
- Jungmi, K.; Inho, C.; Younggeun, L.; Hongsu. Identification of volatile essential oil components of Eoseongcho, fragrance properties and antibacterial activity of fractions-Ⅱ. Fragrant properties and antibacterial activity of fractions by Prep-HPLC. J. Korean Soc. Food Sci. Nutr. 1997, 26, 214–221. [Google Scholar]
- Kumar, R.; Clermont, G.; Vodovotz, Y.; Chow, C.C. The Dynamics of Acute Inflammation. J. Theor. Biol. 2004, 230, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An Inflammation Classification System Using Cytokine Parameters. Scand. J. Immunol. 2021, 93, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.D. Challenges in Allergy Immunology Practice: Solutions Needed for Persistent Patient Problems. Ann. Allergy Asthma Immunol. 2018, 121, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Seo, Y.K.; Park, J.R. Down-Regulation of FcεRI Expression by Houttuynia Cordata Thunb Extract in Human Basophilic KU812F Cells. J. Med. Food 2009, 12, 383–388. [Google Scholar] [CrossRef]
- Satthakarn, S.; Chung, W.; Promsong, A.; Nittayananta, W. Houttuynia Cordata Modulates Oral Innate Immune Mediators: Potential Role of Herbal Plant on Oral Health. Oral Dis. 2015, 21, 512–518. [Google Scholar] [CrossRef]
- Saadat, S.; Beigoli, S.; Khazdair, M.R.; Amin, F.; Boskabady, M.H. Experimental and Clinical Studies on the Effects of Natural Products on Noxious Agents-Induced Lung Disorders, a Review. Front. Nutr. 2022, 9, 867914. [Google Scholar] [CrossRef]
- Shin, S.; Joo, S.S.; Jeon, J.H.; Park, D.; Jang, M.J.; Kim, T.O.; Kim, H.K.; Hwang, B.Y.; Kim, K.Y.; Kim, Y.B. Anti-Inflammatory Effects of a Houttuynia Cordata Supercritical Extract. J. Vet. Sci. 2010, 11, 273–275. [Google Scholar] [CrossRef]
- Zou, M.; Hu, X.; Wang, Y.; Wang, J.; Tang, F.; Liu, Y. Structural Characterization and Anti-Inflammatory Activity of a Pectin Polysaccharide HBHP-3 from Houttuynia Cordata. Int. J. Biol. Macromol. 2022, 210, 161–171. [Google Scholar] [CrossRef]
- Pakyntein, C.L.; Syiem, D.; Thabah, D.; Sunn, S.E. Antioxidant, Anti-Inflammatory and Anti-Hyperglycemic Activity of Aqueous and Methanolic Extract of Houttuynia Cordata: An in Vitro and in Vivo Study. GSC Biol. Pharm. Sci. 2021, 16, 145–154. [Google Scholar] [CrossRef]
- Li, W.; Yang, F.; Zhan, H.; Liu, B.; Cai, J.; Luo, Y.; Zhou, X. Houttuynia Cordata Extract Ameliorates Bladder Damage and Improves Bladder Symptoms via Anti-Inflammatory Effect in Rats with Interstitial Cystitis. Evid.-Based Complement. Altern. Med. 2020, 2020, 9026901. [Google Scholar] [CrossRef]
- Woranam, K.; Senawong, G.; Utaiwat, S.; Yunchalard, S.; Sattayasai, J.; Senawong, T. Anti-Inflammatory Activity of the Dietary Supplement Houttuynia Cordata Fermentation Product in RAW264.7 Cells and Wistar Rats. PLoS ONE 2020, 15, e0230645. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Park, J.H.; Kim, J.Y.; Jeong, H.G. Houttuynia Cordata Water Extract Suppresses Anaphylactic Reaction and IgE-Mediated Allergic Response by Inhibiting Multiple Steps of FcεRI Signaling in Mast Cells. Food Chem. Toxicol. 2009, 47, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.F.; Zheng, L.L.; Liu, Y.; Yu, X. Houttuynia Cordata Thunb Reverses Oxaliplatin-Induced Neuropathic Pain in Rat by Regulating Th17/Treg Balance. Am. J. Transl. Res. 2016, 8, 1609–1614. [Google Scholar]
- Lee, H.J.; Seo, H.S.; Kim, G.J.; Jeon, C.Y.; Park, J.H.; Jang, B.H.; Park, S.J.; Shin, Y.C.; Ko, S.G. Houttuynia Cordata Thunb Inhibits the Production of Pro-Inflammatory Cytokines through Inhibition of the NFκB Signaling Pathway in HMC-1 Human Mast Cells. Mol. Med. Rep. 2013, 8, 731–736. [Google Scholar] [CrossRef]
- Li, W.; Zhou, P.; Zhang, Y.; He, L. Houttuynia Cordata, a Novel and Selective COX-2 Inhibitor with Anti-Inflammatory Activity. J. Ethnopharmacol. 2011, 133, 922–927. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Zhang, Y.Y.; Ou, Y.Y.; Lu, X.X.; Pan, L.Y.; Li, H.; Lu, Y.; Chen, D.F. Houttuynia Cordata Thunb. Polysaccharides Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in Mice. J. Ethnopharmacol. 2015, 173, 81–90. [Google Scholar] [CrossRef]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic Acids and Their Derivatives Are Widely Implicated in Liver Cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Chen, L.; Su, Q.; Wang, Y.; Guo, Z.; Lu, Y.; Liu, B.; Qin, S. Radiation-Induced Intestinal Damage: Latest Molecular and Clinical Developments. Futur. Oncol. 2019, 15, 4105–4118. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, X.; Ling, L.; Li, H.; Ou, Y.; Shi, X.; Lu, Y.; Zhang, Y.; Chen, D. Houttuynia Cordata Polysaccharides Ameliorate Pneumonia Severity and Intestinal Injury in Mice with Influenza Virus Infection. J. Ethnopharmacol. 2018, 218, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, H.; Li, Y.; Dong, N.; Bi, C.; Shan, A.; Wu, Z.; Shi, B. Sodium Houttuyfonate Enhances the Intestinal Barrier and Attenuates Inflammation Induced by Salmonella Typhimurium through the NF-ΚB Pathway in Mice. Int. Immunopharmacol. 2020, 89, 107058. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Pan, Q.; Yang, N. Autophagy and Inflammation Regulation in Acute Kidney Injury. Front. Physiol. 2020, 11, 576463. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Yang, H.-T.; Ho, J.-J.; Yin, M.-C.; Hsu, J.-Y. Houttuynia Cordata Aqueous Extract Attenuated Glycative and Oxidative Stress in Heart and Kidney of Diabetic Mice. Eur. J. Nutr. 2016, 55, 845–854. [Google Scholar] [CrossRef]
- Pan, P.; Wang, Y.J.; Han, L.; Liu, X.; Zhao, M.; Yuan, Y.F. Effects of Sodium Houttuyfonate on Expression of NF-KappaB and MCP-1 in Membranous Glomerulonephritis. J. Ethnopharmacol. 2010, 131, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Ling, L.; Zhang, Y.; Li, H.; Chen, D. Beneficial Effects of Houttuynia Cordata Polysaccharides on “Two-Hit” Acute Lung Injury and Endotoxic Fever in Rats Associated with Anti-Complementary Activities. Acta Pharm. Sin. B 2018, 8, 218–227. [Google Scholar] [CrossRef]
- Shilovskiy, I.P.; Yumashev, K.V.; Nikolsky, A.A.; Vishnyakova, L.I.; Khaitov, M.R. Molecular and Cellular Mechanisms of Respiratory Syncytial Viral Infection: Using Murine Models to Understand Human Pathology. Biochemistry 2021, 86, 290–306. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, H.; Lu, X.X.; Ling, L.J.; Weng, H.B.; Sun, W.; Chen, D.F.; Zhang, Y.Y. Houttuynia Cordata Polysaccharide Alleviated Intestinal Injury and Modulated Intestinal Microbiota in H1N1 Virus Infected Mice. Chin. J. Nat. Med. 2019, 17, 187–197. [Google Scholar] [CrossRef]
- Gao, J.P.; Wang, Y.; Lü, J.; Gu, W.L.; Chen, C.X. Effect of Sodium Houttuyfonate on Myocardial Hypertrophy in Mice and Rats. J. Pharm. Pharmacol. 2009, 61, 677–683. [Google Scholar] [CrossRef]
- Tu, X.; Deng, Y.P.; Chen, J.; Hu, Q.; He, C.S.; Jordan, J.B.; Zhong, S. Screening Study on the Anti-Angiogenic Effects of Traditional Chinese Medicine–Part I: Heat-Clearing and Detoxicating TCM. J. Ethnopharmacol. 2016, 194, 280–287. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Khang, D.T.; Anh, T.T.T.; Trang, P.T.; Xuan, T.D. Antioxidant Properties and Total Phenolic Contents of Various Extracts from Houttuynia Cordata Thunb. Tap Chi Sinh Hoc 2018, 40, 149–154. [Google Scholar] [CrossRef]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia Cordata Thunb. and Its Bioactive Compound 2-Undecanone Significantly Suppress Benzo(a)Pyrene-Induced Lung Tumorigenesis by Activating the Nrf2-HO-1/NQO-1 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jin, C.; Chen, H.; Wang, P.; Yu, M.; Ding, K. Structural Characterization and Anti-A549 Lung Cancer Cells Bioactivity of a Polysaccharide from Houttuynia Cordata. Int. J. Biol. Macromol. 2018, 120, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Koppula, S. Houttuynia Cordata Attenuates Lipid Accumulation via Activation of AMP-Activated Protein Kinase Signaling Pathway in HepG2 Cells. Am. J. Chin. Med. 2014, 42, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Routhier, A.; Astuccio, M.; Lahey, D.; Monfredo, N.; Johnson, A.; Callahan, W.; Partington, A.; Fellows, K.; Ouellette, L.; Zhidro, S.; et al. Pharmacological Inhibition of Rho-Kinase Signaling with Y-27632 Blocks Melanoma Tumor Growth. Oncol. Rep. 2010, 23, 861–867. [Google Scholar] [CrossRef]
- Zhou, N.N.; Tang, J.; Chen, W.D.; Feng, G.K.; Xie, B.F.; Liu, Z.C.; Yang, D.; Zhu, X.F. Houttuyninum, an Active Constituent of Chinese Herbal Medicine, Inhibits Phosphorylation of HER2/Neu Receptor Tyrosine Kinase and the Tumor Growth of HER2/Neu-Overexpressing Cancer Cells. Life Sci. 2012, 90, 770–775. [Google Scholar] [CrossRef]
- Subhawa, S.; Chewonarin, T.; Banjerdpongchai, R. The Effects of Houttuynia Cordata Thunb and Piper Ribesioideswall Extracts on Breast Carcinoma Cell Proliferation, Migration, Invasion and Apoptosis. Molecules 2020, 25, 1196. [Google Scholar] [CrossRef]
- Sandip, B.J.J.M.A.P.S. Medicinal Plants with Anti-HIV Potential. Clin. Exp. Pharmacol. Physiol. 2003, 25, 427–440. [Google Scholar]
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal Effects of the Steam Distillate from Houttuynia Cordata and Its Components on HSV-1, Influenza Virus, and HIV. Planta Med. 1995, 61, 237–241. [Google Scholar] [CrossRef]
- Lau, K.M.; Lee, K.M.; Koon, C.M.; Cheung, C.S.F.; Lau, C.P.; Ho, H.M.; Lee, M.Y.H.; Au, S.W.N.; Cheng, C.H.K.; Lau, C.B.S.; et al. Immunomodulatory and Anti-SARS Activities of Houttuynia Cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, J.H.; Lee, C.H.; Ahn, Y.J.; Song, J.H.; Baek, S.H.; Kwon, D.H. Antiviral Activity of Quercetin 7-Rhamnoside against Porcine Epidemic Diarrhea Virus. Antivir. Res. 2009, 81, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, D.K.; Guo, Y.M.; Feng, W.W.; Dong, Q.; Zhang, C.E.; Zhou, Y.F.; Liu, Y.; Wang, J.B.; Zhao, Y.L.; et al. Screening and Evaluation of Commonly-Used Anti-Influenza Chinese Herbal Medicines Based on Anti-Neuraminidase Activity. Chin. J. Nat. Med. 2016, 14, 794–800. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.-X.; Li, E. Houttuynia Cordata Blocks HSV Infection through Inhibition of NF-ΚB Activation. Antivir. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, G.; Li, J.; Yang, Q.; Ren, X. In Vitro and in Vivo Effects of Houttuynia Cordata on Infectious Bronchitis Virus. Avian Pathol. 2011, 40, 491–498. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential Roles of Medicinal Plants for the Treatment of Viral Diseases Focusing on COVID-19: A Review. Phyther. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef]
- Das, S.K.; Mahanta, S.; Tanti, B.; Tag, H.; Hui, P.K. Identification of Phytocompounds from Houttuynia Cordata Thunb. as Potential Inhibitors for SARS-CoV-2 Replication Proteins through GC–MS/LC–MS Characterization, Molecular Docking and Molecular Dynamics Simulation. Mol. Divers. 2022, 26, 365–388. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-Herpes Simplex Virus Type 1 Activity of Houttuynoid A, a Flavonoid from Houttuynia Cordata Thunb. Antivir. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral Effects of Houttuynia Cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)-A Human Norovirus Surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of Antiviral Activities of Houttuynia Cordata Thunb. Extract, Quercetin, Quercetrin and Cinanserin on Murine Coronavirus and Dengue Virus Infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Lin, T.Y.; Liu, Y.C.; Jheng, J.R.; Tsai, H.P.; Jan, J.T.; Wong, W.R.; Horng, J.T. Anti-Enterovirus 71 Activity Screening of Chinese Herbs with Anti-Infection and Inflammation Activities. Am. J. Chin. Med. 2009, 37, 143–158. [Google Scholar] [CrossRef]
- Lelešius, R.; Karpovaite, A.; Mickiene, R.; Drevinskas, T.; Tiso, N.; Ragažinskiene, O.; Kubiliene, L.; Maruška, A.; Šalomskas, A. In Vitro Antiviral Activity of Fifteen Plant Extracts against Avian Infectious Bronchitis Virus. BMC Vet. Res. 2019, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Duan, Q.; Li, F.; Shao, J.; Cheng, H.; Wu, D. Sodium Houttuyfonate and EDTA-Na2 in Combination Effectively Inhibits Pseudomonas Aeruginosa, Staphylococcus Aureus and Candida Albicans in Vitro and in Vivo. Bioorganic Med. Chem. Lett. 2015, 25, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Cheng, H.; Duan, Q.; Huang, W. Sodium Houttuyfonate Inhibits Biofilm Formation and Alginate Biosynthesis-Associated Gene Expression in a Clinical Strain of Pseudomonas Aeruginosa in Vitro. Exp. Ther. Med. 2015, 10, 753–758. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, D.H.; Ju, L.J.; Lee, J.J.; Han, D.Y.; Lee, W.M.; Jung, W.C.; Min, W.G.; Won, C.G.; Rhee, M.H.; et al. Biological and Antibacterial Activities of the Natural Herb Houttuynia Cordata Water Extract against the Intracellular Bacterial Pathogen Salmonella within the RAW 264.7 Macrophage. Biol. Pharm. Bull. 2008, 31, 2012–2017. [Google Scholar] [CrossRef]
- Chen, H.; Sha, X.; Luo, Y.; Chen, J.; Li, X.; Wang, J.; Cao, G.; Peng, X. Acute and Subacute Toxicity Evaluation of Houttuynia Cordata Ethanol Extract and Plasma Metabolic Profiling Analysis in Both Male and Female Rats. J. Appl. Toxicol. 2021, 41, 2068–2082. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Duangjitcharoen, Y.; Kesika, P.; Sirilun, S.; Chaiyasut, K.; Peerajan, S. Assessment of Subchronic Toxicity of Fermented Houttuynia Cordata Thunb. Using Rodent Model System. Asian J. Pharm. Clin. Res. 2018, 11, 307–311. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic Potentials of Houttuynia Cordata Thunb. against Inflammation and Oxidative Stress: A Review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best Practice in Research–Overcoming Common Challenges in Phytopharmacological Research. J. Ethnopharmacol. 2020, 246, 112230. [Google Scholar] [CrossRef]


| Activities of Different H. cordata Extracts | * DPPH IC50 (mg/mL) | * ABTS IC50 (mg/mL) | * LPI% |
|---|---|---|---|
| Hexane | NA | 6.14 ± 0.67 a | 67.43 ± 1.28 c |
| Chloroform | NA | 4.64 ± 0.67 b | 81.80 ± 0.91 b |
| Acetonitrile | NA | 0.59 ± 0.01 d | 83.69 ± 0.50 b |
| Ethanol | 0.10 ± 0.01 b | 0.42 ± 0.01 d | 85.89 ± 0.82 b |
| Methanol | 0.05 ± 0.00 c | 0.23 ± 0.00 d | 86.61 ± 0.76 ab |
| Water | 0.22 ± 0.01 a | 2.19 ± 0.03 c | 63.35 ± 5.43 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review. Pharmaceuticals 2022, 15, 1079. https://doi.org/10.3390/ph15091079
Rafiq S, Hao H, Ijaz M, Raza A. Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review. Pharmaceuticals. 2022; 15(9):1079. https://doi.org/10.3390/ph15091079
Chicago/Turabian StyleRafiq, Shahzad, Haihong Hao, Muhammad Ijaz, and Ahmed Raza. 2022. "Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review" Pharmaceuticals 15, no. 9: 1079. https://doi.org/10.3390/ph15091079
APA StyleRafiq, S., Hao, H., Ijaz, M., & Raza, A. (2022). Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review. Pharmaceuticals, 15(9), 1079. https://doi.org/10.3390/ph15091079