Polyhydric Stigmastane-Type Steroids Derivative from Vernonia amygdalina and Their Anti-Neuroinflammatory Activity
Abstract
:1. Introduction
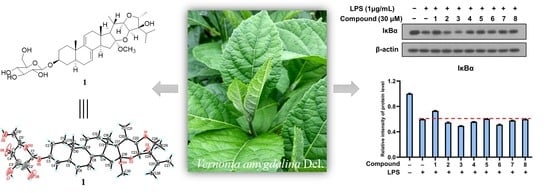
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. X-ray Diffraction Analysis of Compound 1
3.5. Computational Electronic Circular Dichroism (ECD) Spectrum Calculation Method
3.6. Snatzke’s Method to Identify the Absolute Configuration of the Vic-Diols in 7
3.7. Cell Culture
3.8. Cell Proliferation Assay
3.9. Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Wei, W.Y.; Xu, H.X. Drug discovery is an eternal challenge for the biomedical sciences. Acta Mater. Med. 2022, 1, 1–3. [Google Scholar] [CrossRef]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef] [PubMed]
- Ijeh, I.I.; Ejike, C.E.C.C. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J. Med. Plants Res. 2011, 5, 1051–1061. [Google Scholar] [CrossRef]
- Oyugi, D.A.; Luo, X.; Lee, K.S.; Hill, B.; Izevbigie, E.B. Activity Markers of the Anti-Breast Carcinoma Cell Growth Fractions of Vernonia amygdalina Extracts. Exp. Biol. Med. 2009, 234, 410–417. [Google Scholar] [CrossRef]
- Jisaka, M.; Ohigashi, H.; Takegawa, K.; Huffman, M.A.; Koshimizu, K. Antitumoral and Antimicrobial Activities of Bitter Sesquiterpene Lactones of Vernonia amygdalina, a Possible Medicinal Plant Used by Wild Chimpanzees. Biosci. Biotechnol. Biochem. 1993, 57, 833–834. [Google Scholar] [CrossRef]
- Izevbigie, E.B. Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp. Biol. Med. 2003, 228, 293–298. [Google Scholar] [CrossRef]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Antioxidant Constituents in Vernonia amygdalina Leaves. Pharm. Biol. 2007, 45, 195–199. [Google Scholar] [CrossRef]
- Fasakin, C.F.; Udenigwe, C.C.; Aluko, R.E. Antioxidant properties of chlorophyll-enriched and chlorophyll-depleted polyphenolic fractions from leaves of Vernonia amygdalina and Gongronema latifolium. Food Res. Int. 2011, 44, 2435–2441. [Google Scholar] [CrossRef]
- Tona, L.; Cimanga, R.K.; Mesia, K.; Musuamba, C.T.; Bruyne, T.D.; Apers, S.; Hernans, N.; Miert, S.V.; Pieters, L.; Totté, J.; et al. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J. Ethnopharmacol. 2004, 93, 27–32. [Google Scholar] [CrossRef]
- Atangwho, I.J.; Egbung, G.E.; Ahmad, M.; Yam, M.F.; Asmawia, M.Z. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013, 141, 3428–3434. [Google Scholar] [CrossRef]
- Ademola, I.O.; Eloff, J.N. Anthelminthic activity of acetone extract and fractions of Vernonia amygdalina against Haemonchus contortus eggs and larvae. Trop. Anim. Health Prod. 2011, 43, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, A.; Melaku, Y. Antibacterial and Antioxidant Compounds from the Flower Extracts of Vernonia amygdalina. Adv. Pharmacol. Sci. 2018, 2018, 4083736. [Google Scholar] [CrossRef] [PubMed]
- Igile, G.O.; Oleszek, W.; Burda, S.; Jurzysta, M. Nutritional Assessment of Vernonia amygdalina Leaves in Growing Mice. J. Agric. Food Chem. 2002, 43, 2162–2166. [Google Scholar] [CrossRef]
- Ganjian, I.; Kubo, I.; Fludzinski, P. Insect antifeedant elemanolide lactones from Vernonia amygdalina. Phytochemistry 1983, 22, 2525–2526. [Google Scholar] [CrossRef]
- Green, P.W.C.; Belmain, S.R.; Ndakidemi, P.A.; Farrell, I.W.; Stevenson, P.C. Insecticidal activity of Tithonia diversifolia and Vernonia amygdalina. Ind. Crop. Prod. 2017, 110, 15–21. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Wu, X.; Zhang, S.; Gao, X.; Li, F.; Zhu, X.; Chen, Q. Steroidal Saponins from Vernonia amygdalina Del. and Their Biological Activity. Molecules 2018, 23, 579. [Google Scholar] [CrossRef]
- Zhao, M.-L.; Shan, S.-J.; Tao, R.; Cui, L.-T.; Li, Q.-R.; Luo, J.; Li, Y. Stigmastane-type steroid saponins from the leaves of Vernonia amygdalina Del. Fitoterapia 2021, 150, 104838. [Google Scholar] [CrossRef]
- Nguyen, T.X.T.; Dang, D.L.; Ngo, V.Q.; Trinh, T.C.; Trinh, Q.N.; Do, T.D.; Thanh, T.T.T. Anti-inflammatory activity of a new compound from Vernonia amygdalina. Nat. Prod. Res. 2020, 35, 5160–5165. [Google Scholar] [CrossRef]
- Anh HL, T.; Vinh, L.B.; Lien, L.T.; Cuong, P.V.; Arai, M.; Ha, T.P.; Lin, H.N.; Dat, T.T.H.; Cuong, L.V.; Kim, Y.H. In vitro study on α-amylase and α-glucosidase inhibitory activities of a new stigmastane-type steroid saponin from the leaves of Vernonia amygdalina. Nat. Prod. Res. 2021, 35, 873–879. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.S.; Tian, W.J.; Wang, G.H.; Zhang, X.; Zhou, M.; Zeng, D.Q.; Liu, X.Z.; Yao, X.S.; Zhang, Y.W.; Chen, H.F. Phenolic Glycosides from the Roots of Ficus hirta Vahl. and Their Antineuroinflammatory Activities. J. Agric. Food Chem. 2020, 68, 4196–4204. [Google Scholar] [CrossRef] [PubMed]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R. Pretreatment with Kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.-j.; Park, S.K.; Park, J.-H.; Jeong, H.-R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.-S. Donepezil Regulates LPS and Aβ-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Liu, X.Z.; Tian, W.J.; Wang, G.H.; Xu, Q.N.; Zhou, M.; Gao, S.; Qiu, D.R.; Jiang, X.; Sun, C.L.; Ding, R.; et al. Stigmastane-type steroids with unique conjugated Δ7,9(11) diene and highly oxygenated side chains from the twigs of Vernonia amygdalina. Phytochemistry 2019, 158, 67–76. [Google Scholar] [CrossRef]
- Bari, L.D.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-κB system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef]
- Wei, J.; Feng, J. Signaling Pathways Associated with Inflammatory Bowel Disease. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. BBA-Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]






| No. | 1 a | 2 a | 3 b | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 1α | 35.3 | 1.27, m | 35.3 | 1.21, overlap | 35.3 | 1.23, overlap | 35.2 | 1.24, m |
| 1β | 1.87, m | 1.84, overlap | 1.83, m | 1.87, m | ||||
| 2α | 30.5 | 2.18, m | 30.5 | 2.16, br d (8.0) | 30.5 | 2.15, overlap | 30.4 | 2.15, m |
| 2β | 1.73, m | 1.72, m | 1.70, m | 1.72, br d (13.3) | ||||
| 3 | 77.5 | 3.99, overlap | 77.5 | 3.98, m | 77.4 | 3.97, m | 77.4 | 3.98, m |
| 4α | 34.9 | 2.07, m | 34.9 | 2.07, br d (12.4) | 34.9 | 2.03, br d (11.4) | 34.8 | 2.04, m |
| 4β | 1.43, d (11.8) | 1.43, m | 1.40, q (11.7) | 1.41, q (12.1) | ||||
| 5 | 39.7 | 1.39, m | 39.7 | 1.38, m | 39.6 | 1.32, m | 39.5 | 1.34, m |
| 6α | 30.5 | 1.84, m | 30.5 | 1.85, m | 30.5 | 1.80, m | 30.5 | 1.81, m |
| 6β | 1.84, m | 1.85, m | 1.80, m | 1.81, m | ||||
| 7 | 121.6 | 5.42, br s | 121.5 | 5.44, br s | 121.9 | 5.31, br s | 121.7 | 5.31, br s |
| 8 | 135.2 | 135.3 | 135.5 | 135.6 | ||||
| 9 | 144.5 | 144.4 | 144.6 | 144.4 | ||||
| 10 | 36.7 | 36.6 | 36.5 | 36.5 | ||||
| 11 | 119.0 | 5.45, br d (5.8) | 119.2 | 5.46, overlap | 118.7 | 5.42, br s | 118.8 | 5.43, br d (6.8) |
| 12α | 43.6 | 2.11, d (6.3) | 43.3 | 2.37, m | 42.2 | 2.32, overlap | 43.1 | 2.27, br d (16.9) |
| 12β | 2.15, overlap | 2.44, m | 2.32, overlap | 2.34, dd (17.2, 6.4) | ||||
| 13 | 42.0 | 42.2 | 43.4 | 43.4 | ||||
| 14 | 47.3 | 2.35, br d (13.5) | 47.5 | 2.45, m | 49.6 | 2.54, m | 49.6 | 2.52, m |
| 15α | 32.8 | 2.43, dd (12.8, 5.6) | 33.1 | 2.50, m | 34.5 | 1.89, m | 34.6 | 1.86, m |
| 15β | 1.51, m | 1.61, t (12.9) | 2.10, overlap | 2.06, m | ||||
| 16 | 111.0 | 111.5 | 79.5 | 5.55, br s | 80.1 | 5.40, t (7.1) | ||
| 17 | 61.6 | 1.34, d (11.8) | 54.5 | 2.26, m | 57.0 | 2.32, overlap | 61.0 | 2.19, dd (10.7, 6.2) |
| 18 | 13.7 | 0.60, s | 14.2 | 0.71, s | 13.3 | 0.66, s | 13.0 | 0.64, s |
| 19 | 19.9 | 0.81, s | 19.8 | 0.82, s | 19.8 | 0.79, s | 19.8 | 0.81, s |
| 20 | 33.7 | 2.17, overlap | 41.0 | 2.35, m | 40.6 | 2.73, br s | 35.4 | 2.48, m |
| 21a | 18.2 | 1.15, d (6.3) | 60.2 | 4.43, m | 61.8 | 4.25, m | 16.2 | 1.45, d (5.6) |
| 21b | 3.89, m | 4.19, m | ||||||
| 22 | 80.1 | 3.97, dd (10.3, 7.6) | 74.8 | 4.71, m | 79.0 | 4.52, br d (6.4) | 80.4 | 4.29, m |
| 23 | 76.1 | 4.18, d (7.4) | 76.1 | 4.24, br d (6.8) | 76.9 | 4.36, m | 76.0 | 4.35, t (5.6) |
| 24 | 83.3 | 83.5 | 83.5 | 82.7 | ||||
| 25 | 33.5 | 1.86, m | 33.5 | 1.89, m | 32.8 | 1.89, m | 32.4 | 1.94, m |
| 26 | 17.9 | 1.24, d (6.7) | 17.9 | 1.26, d (5.9) | 18.1 | 1.27, d (6.4) | 17.9 | 1.19, d (5.9) |
| 27 | 18.2 | 1.07, d (6.5) | 18.2 | 1.08, d (5.7) | 18.3 | 1.10, d (6.1) | 18.3 | 1.12, d (6.4) |
| 28 | 82.2 | 4.36, overlap | 82.2 | 4.40, m | 81.8 | 4.39, m | 81.2 | 4.41, m |
| 29 | 17.3 | 1.21, d (6.7) | 17.2 | 1.20, d (5.8) | 15.3 | 1.24, d (6.5) | 15.4 | 1.29, d (6.7) |
| 16-OMe | 50.5 | 3.35, s | 50.6 | 3.37, s | ||||
| 16-OAc | 171.0 | 171.0 | ||||||
| 21.8 | 2.13, s | 21.8 | 2.15, s | |||||
| 1′ | 102.8 | 5.04, d (7.7) | 102.8 | 5.02, overlap | 102.7 | 5.03, overlap | 102.7 | 5.02, d (7.7) |
| 2′ | 75.8 | 4.07, t (7.9) | 75.7 | 4.07, m | 75.8 | 4.07, m | 75.7 | 4.05, t (7.9) |
| 3′ | 79.1 | 4.31, overlap | 79.0 | 4.31, m | 79.1 | 4.31, m | 79.0 | 4.31, m |
| 4′ | 72.1 | 4.29, overlap | 72.1 | 4.28, m | 72.2 | 4.28, m | 72.1 | 4.28, m |
| 5′ | 78.9 | 4.01, m | 78.8 | 4.01, m | 78.9 | 4.01, m | 78.9 | 4.00, m |
| 6′ | 63.3 | 4.59, br d (11.5) | 63.2 | 4.59, br d (11.3) | 63.3 | 4.60, br d (11.6) | 63.2 | 4.58, br d (11.5) |
| 4.43, dd (11.6, 5.1) | 4.43, m | 4.42, dd (11.8, 5.2) | 4.41, m | |||||
| No. | 5 a | 6 a | 7 a | 8 a | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 1α | 35.3 | 1.24, dd (13.6,3.4) | 35.3 | 1.26, td (13.3, 3.0) | 35.9 | 1.34, td (13.6, 3.5) | 35.6 | 1.39, t (13.6) |
| 1β | 1.86, dt (13.4, 3.1) | 1.89, overlap | 1.99, overlap | 1.97, m | ||||
| 2α | 30.5 | 2.14, m | 30.5 | 2.15, m | 32.2 | 1.84, m | 33.0 | 2.12, m |
| 2β | 1.71, m | 1.72, br d (12.8) | 1.48, m | 1.77, m | ||||
| 3 | 77.4 | 3.95, m | 77.4 | 3.97, m | 71.4 | 3.51, m | 70.6 | 3.84, m |
| 4α | 34.8 | 2.01, m | 34.8 | 2.03, br d (11.3) | 38.4 | 1.70, m | 39.2 | 1.97, m |
| 4β | 1.41, q (12.5) | 1.41, q (12.0) | 1.29, m | 1.60, q (12.5) | ||||
| 5 | 39.5 | 1.30, overlap | 39.6 | 1.32, m | 40.6 | 1.42, m | 40.0 | 1.49, overlap |
| 6α | 30.6 | 1.80, m | 30.6 | 1.82, m | 30.9 | 1.91, m | 30.7 | 1.89, m |
| 6β | 1.80, m | 1.82, m | 1.91, m | 1.89, m | ||||
| 7 | 121.3 | 5.39, br s | 120.9 | 5.43, br s | 122.1 | 5.39, br s | 121.5 | 5.45, br s |
| 8 | 136.5 | 136.6 | 136.3 | 136.5 | ||||
| 9 | 144.5 | 144.7 | 145.2 | 144.7 | ||||
| 10 | 36.5 | 36.5 | 37.0 | 36.0 | ||||
| 11 | 119.0 | 5.43, br d (6.0) | 119.2 | 5.46, br d (6.0) | 119.3 | 5.52, br d (6.4) | 119.0 | 5.51, br d (5.4) |
| 12α | 43.3 | 2.29, br d (16.8) | 43.5 | 2.23, br d (16.6) | 43.6 | 2.25, br d (17.1) | 43.4 | 2.34, br d (17.0) |
| 12β | 2.36, dd (17.2, 6.6) | 2.39, dd (17.2, 6.6) | 2.35, dd (17.2, 6.7) | 2.41, dd (17.2, 6.3) | ||||
| 13 | 44.3 | 43.4 | 44.0 | 44.3 | ||||
| 14 | 49.6 | 2.77, m | 50.5 | 2.20, overlap | 50.1 | 2.44, m | 49.7 | 2.82, m |
| 15α | 37.1 | 2.08, m | 37.2 | 1.88, overlap | 34.8 | 1.70, m | 37.2 | 2.10, m |
| 15β | 2.14, m | 2.54, dt (12.8, 7.7) | 1.97, m | 2.18, mk | ||||
| 16 | 76.5 | 4.58, overlap | 72.3 | 4.87, br s | 81.0 | 5.00, t (7.0) | 76.5 | 4.59, br s |
| 17 | 65.2 | 2.12, overlap | 60.1 | 1.97, dd (10.8, 7.4) | 61.3 | 1.85, m | 65.3 | 2.15, m |
| 18 | 13.4 | 0.69, s | 13.3 | 1.05, s | 12.9 | 0.61, s | 13.5 | 0.73, s |
| 19 | 19.8 | 0.83, s | 20.0 | 0.86, s | 19.9 | 0.91, s | 20.1 | 0.97, s |
| 20 | 35.5 | 2.60, m | 31.7 | 3.10, m | 35.4 | 2.06, m | 35.6 | 2.62, m |
| 21a | 16.6 | 1.47, d (6.7) | 15.8 | 1.52, d (6.7) | 15.7 | 1.07, d (6.7) | 16.6 | 1.50, d (6.7) |
| 21b | ||||||||
| 22 | 81.2 | 4.84, br s | 82.0 | 4.60, br d (6.0) | 80.7 | 3.93, dd (7.1, 1.4) | 81.3 | 4.87, br s |
| 23 | 76.3 | 4.51, t (6.0) | 75.9 | 4.44, t (4.9) | 76.4 | 4.03, d (7.1) | 76.3 | 4.52, t (5.3) |
| 24 | 83.0 | 82.6 | 83.1 | 83.1 | ||||
| 25 | 32.4 | 1.95, m | 32.3 | 1.92, m | 32.9 | 1.81, m | 32.5 | 1.95, m |
| 26 | 18.1 | 1.21, d (6.8) | 17.9 | 1.14, d (6.8) | 17.4 | 0.95, d (6.4) | 18.1 | 1.22, d (6.8) |
| 27 | 18.4 | 1.12, d (6.6) | 18.5 | 1.10, d (6.5) | 17.9 | 0.94, d (6.4) | 18.5 | 1.13, d (6.5) |
| 28 | 81.2 | 4.42, q (6.7) | 81.3 | 4.43, q (7.1) | 81.8 | 3.97, q (6.7) | 81.3 | 4.43, q (6.4) |
| 29 | 15.5 | 1.12, d (6.7) | 15.6 | 1.28, d (6.7) | 15.1 | 1.12, d (6.7) | 15.5 | 1.33, d (6.7) |
| 16-OAc | 172.7 | |||||||
| 21.5 | 2.00, s | |||||||
| 1′ | 102.6 | 5.02, overlap | 102.6 | 5.02, d (7.7) | ||||
| 2′ | 75.7 | 4.04, t (8.0) | 75.7 | 4.04, t (8.1) | ||||
| 3′ | 79.0 | 4.29, m | 79.0 | 4.29, m | ||||
| 4′ | 72.1 | 4.27, m | 72.1 | 4.26, m | ||||
| 5′ | 78.8 | 3.98, m | 78.8 | 3.99, m | ||||
| 6′ | 63.2 | 4.57, br d (10.9) | 63.2 | 4.57, br d (10.9) | ||||
| 4.40, m | 4.40, dd (11.6, 5.2) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, M.; He, S.; Xu, Q.; Du, C.; Zhu, H.; Lin, T.; Wang, G.; Tian, W.; Chen, H. Polyhydric Stigmastane-Type Steroids Derivative from Vernonia amygdalina and Their Anti-Neuroinflammatory Activity. Pharmaceuticals 2022, 15, 1160. https://doi.org/10.3390/ph15091160
Liu X, Zhou M, He S, Xu Q, Du C, Zhu H, Lin T, Wang G, Tian W, Chen H. Polyhydric Stigmastane-Type Steroids Derivative from Vernonia amygdalina and Their Anti-Neuroinflammatory Activity. Pharmaceuticals. 2022; 15(9):1160. https://doi.org/10.3390/ph15091160
Chicago/Turabian StyleLiu, Xiangzhong, Mi Zhou, Shoulun He, Qiannan Xu, Chunchun Du, Honghong Zhu, Ting Lin, Guanghui Wang, Wenjing Tian, and Haifeng Chen. 2022. "Polyhydric Stigmastane-Type Steroids Derivative from Vernonia amygdalina and Their Anti-Neuroinflammatory Activity" Pharmaceuticals 15, no. 9: 1160. https://doi.org/10.3390/ph15091160
APA StyleLiu, X., Zhou, M., He, S., Xu, Q., Du, C., Zhu, H., Lin, T., Wang, G., Tian, W., & Chen, H. (2022). Polyhydric Stigmastane-Type Steroids Derivative from Vernonia amygdalina and Their Anti-Neuroinflammatory Activity. Pharmaceuticals, 15(9), 1160. https://doi.org/10.3390/ph15091160