Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile
Abstract
:1. Introduction
2. Results
2.1. Characterization of Gamma Conglutin and Lupanine
2.2. Acute Treatment Effects of Different Cγ and Lupanine Doses Evaluated by Oral Glucose Tolerance Test (OGTT)
2.2.1. In Healthy Rats, an Enhanced Antidiabetic Effect Was Elicited by the Combination of Cγ (28 mg/kg BW) + Lupanine (20 mg/kg BW)
2.2.2. In Diabetic Rats, the Glycemia Reduction Effect of the Treatment Combination of Cγ + Lupanine Was Similar to the Pharmacological Effect of a Conventional Treatment (Metformin + Glibenclamide)
2.3. Chronic Treatment Effects
2.3.1. Diabetic Rats Exhibited Body Weight Loss after 7 Days of Treatment (28 mg/kg BW Cγ + 20 mg/kg BW Lupanine)
2.3.2. Biochemical Parameters in Diabetic Rats Treated with Cγ + Lupanine
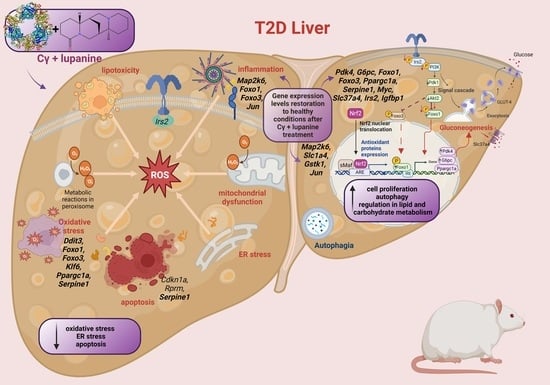
2.4. Influence of the Cγ + Lupanine Combination on the Liver Gene Expression Profile of Diabetic Rats
Transcriptome Analysis Console v. 4.0.2
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.1.1. Extraction and Characterization of Bioactive Compounds from Defatted Lupinus Albus Flour
Isolation of the Quinolizidine Alkaloid, Lupanine
Characterization and Detection of Alkaloids by Thin-Layer Chromatography (TLC)
Gamma Conglutin (Cγ) Extraction
Characterization of Cγ by SDS-PAGE
4.2. Preparation of Protein in Solution with Alkaloid
4.3. Animals
4.4. Study Design
4.4.1. Oral Glucose Tolerance Tests to Screen Cγ and Lupanine Combined Doses and Its Acute Treatment Effects
Oral Glucose Tolerance Test (OGTT)
Delta Glucose Curves
Delta Glucose AUC Values
4.4.2. Chronic Treatment Effects
4.5. T2D Model
4.6. Blood Collection
4.7. Biochemical Parameters
4.8. RNA Extraction
4.9. DNA Microarray
4.10. Bioinformatic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrea, L.; Vetrani, C.; Caprio, M.; El Ghoch, M.; Frias-Toral, E.; Mehta, R.J.; Mendez, V.; Moriconi, E.; Paschou, S.A.; Pazderska, A.; et al. Nutritional management of type 2 diabetes in subjects with obesity: An international guideline for clinical practice. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, A.; Alruwaili, N.K.; Panda, D.S.; Imam, S.S.; Alharbi, K.S.; Afzal, M.; Shalaby, K.; Kazmi, I.; Alshehri, S. Potential of natural bioactive compounds in management of diabetes: Review of preclinical and clinical evidence. Curr. Pharmacol. Rep. 2021, 7, 107–122. [Google Scholar] [CrossRef]
- Janusz, P. White lupin (Lupinus albus L.)–nutritional and health values in human nutrition–a review. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Czubinski, J.; Barciszewski, J.; Gilski, M.; Szpotkowski, K.; Debski, J.; Lampart-Szczapa, E.; Jaskolski, M. Structure of γ-conglutin: Insight into the quaternary structure of 7S basic globulins from legumes. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Bringans, S.; Johnson, S.; Pareek, V.; Utikar, R. Reverse phase HPLC method for detection and quantification of lupin seed γ-conglutin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1063, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.D.; Galanti, E.; Capraro, J.; Magni, C.; Scarafoni, A. Lupinus albus γ-Conglutin, a Protein Structurally Related to GH12 Xyloglucan-Specific Endo-Glucanase Inhibitor Proteins (XEGIPs), Shows Inhibitory Activity against GH2 β-Mannosidase. Int. J. Mol. Sci. 2020, 21, 7305. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin gamma, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Administration of Lupinus albus gamma conglutin (Cγ) to n5 STZ rats augmented Ins-1 gene expression and pancreatic insulin content. Plant Foods Hum. Nutr. 2014, 69, 241–247. [Google Scholar] [CrossRef]
- González-Santiago, A.E.; Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupinus albus Conglutin Gamma Modifies the Gene Expressions of Enzymes Involved in Glucose Hepatic Production In Vivo. Plant Foods Hum. Nutr. 2017, 72, 134–140. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of white lupin seed, a naturally isoflavone-poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Fontanari, G.G.; Batistuti, J.P.; Cruz, R.J.D.; Saldiva, P.H.N.; Arêas, J.A.G. Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C. Hypocholesterolaemic Effects of Lupin Protein and Pea Protein/fibre Combinations in Moderately Hypercholesterolaemic Individuals. Br. J. Nutr. 2012, 107, 1176–1183. [Google Scholar] [CrossRef]
- Radtke, J.; Schutkowski, A.; Brandsch, C.; Hirche, F.; Hasenkopf, K.; Stangl, G.I. Isolated Conglutin γ from Lupin, but not Phytate, Lowers Serum Cholesterol Without Influencing Vascular Lesion Development in the ApoE-deficient Mouse Model. Plant Foods Hum. Nutr. 2015, 70, 113–118. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Calabresi, L.; Arnoldi, A. Lupin protein exerts cholesterol-lowering effects targeting PCSK9: From clinical evidences to elucidation of the in vitro molecular mechanism using HepG2 cells. J. Funct. Foods 2016, 23, 230–240. [Google Scholar] [CrossRef]
- Pavanello, C.; Lammi, C.; Ruscica, M.; Bosisio, R.; Mombelli, G.; Zanoni, C.; Calabresi, L.; Sirtori, C.R.; Magni, P.; Arnoldi, A. Effects of a lupin protein concentrate on lipids, blood pressure and insulin resistance in moderately dyslipidaemic patients: A randomised controlled trial. J. Funct. Foods 2017, 37, 8–15. [Google Scholar] [CrossRef]
- Wojciechowicz, T.; Czubiński, J.; Billert, M.; Półciennik, A.; Nowak, K.W.; Skrzypski, M. Suppressive effects of γ-conglutin on differentiation of 3T3-L1 preadipocytes. Int. J. Food Sci. Technol. 2018, 53, 2624–2630. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Alché, J.D.; Morales-Santana, S.; Clemente, A.; Jimenez-Lopez, J.C. Narrow-Leafed Lupin (Lupinus angustifolius L.) Seeds Gamma-Conglutin is an Anti-Inflammatory Protein Promoting Insulin Resistance Improvement and Oxidative Stress Amelioration in PANC-1 Pancreatic Cell-Line. Antioxidants 2019, 9, 12. [Google Scholar] [CrossRef]
- Guzmán, T.J.; Vargas-Guerrero, B.; García-López, P.M.; Gurrola-Díaz, C.M. Analysis of hepatic transcriptome modulation exerted by γ-conglutin from lupins in a streptozotocin-induced diabetes model. Gene 2020, 761, 145036. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J. Fluorescence polarization as an approach to study the molecular interaction between lupin seed γ-conglutin and insulin. Food Biosci. 2021, 42, 101073. [Google Scholar] [CrossRef]
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, A.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-mimetic action of conglutin-γ, a lupin seed protein, in mouse myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Parolari, A.; Magni, C.; Duranti, M. Lupin seed γ-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br. J. Nutr. 2012, 107, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Wink, M.; Heinen, H.J.; Vogt, H.; Schiebel, H.M. Cellular localization of quinolizidine alkaloids by laser desorption mass spectrometry (LAMMA 1000). Plant Cell Rep. 1984, 3, 230–233. [Google Scholar] [CrossRef]
- Wink, M.; Meißner, C.; Witte, L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 1995, 38, 139–153. [Google Scholar] [CrossRef]
- El-Shazly, A.; Ateya, A.-M.M.; Wink, M. Quinolizidine Alkaloid Profiles of Lupinus varius orientalis, L. albus albus, L. hartwegii, and L. densiflorus. Z. Nat. C 2001, 56, 21–30. [Google Scholar] [CrossRef]
- Ganzera, M.; Krüger, A.; Wink, M. Determination of quinolizidine alkaloids in different Lupinus species by NACE using UV and MS detection. J. Pharm. Biomed. Anal. 2010, 53, 1231–1235. [Google Scholar] [CrossRef]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Chemical Defense of Leguminosae. Are Quinolizidine Alkaloids Part of the Antimicrobial Defense System of Lupins? Z. Nat. C 1984, 39, 548–552. [Google Scholar] [CrossRef]
- Tyski, S.; Markiewicz, M.; Gulewicz, K.; Twardowski, T. The effect of lupin alkaloids and ethanol extracts from seeds of Lupinus angustifolius on selected bacterial strains. J. Plant Physiol. 1988, 133, 240–242. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.W.; Cofta, G.; Mazela, B.; Gobakken, L.R. Fungistatic activity of quinolizidine and bisquinolizidine alkaloids against A. niger. In The International Research Group on Wood Protection. In Proceedings of the 47th IRG Annual Meeting, Lisbon, Portugal, 15–19 May 2016. [Google Scholar]
- Kinghorn, A.D.; Balandrin, M.F. Quinolizidine alkaloids of the Leguminosae: Structural types, analysis, chemotaxonomy, and biological activities. Alkaloids Chem. Biol. Perspect. 1984, 2, 105–148. [Google Scholar]
- Wink, M. Quinolizidine Alkaloids: Biochemistry, Metabolism, and Function in Plants and Cell Suspension Cultures. Planta Med. 1987, 53, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Ikewuchi, C.C.; Ikewuchi, J.C.; Ifeanacho, M.O. Phytochemical composition of Tridax procumbens Linn leaves: Potential as a functional food. Nutr. Food Sci. 2015, 6, 992. [Google Scholar] [CrossRef] [Green Version]
- Petterson, D.S.; Ellis, Z.L.; Harris, D.J.; Spadek, Z.E. Acute toxicity of the major alkaloids of cultivated Lupinus angustifolius seed to rats. J. Appl. Toxicol. 1987, 7, 51–53. [Google Scholar] [CrossRef]
- Wiedemann, M.; Gurrola-Díaz, C.M.; Vargas-Guerrero, B.; Wink, M.; García-López, P.M.; Düfer, M. Lupanine Improves Glucose Homeostasis by Influencing KATP Channels and Insulin Gene Expression. Molecules 2015, 20, 19085–19100. [Google Scholar] [CrossRef]
- Sandoval-Muñíz, R.D.J.; Vargas-Guerrero, B.; Guzmán, T.J.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupin gamma conglutin protein: Effect on Slc2a2, Gck and Pdx-1 gene expression and GLUT2 levels in diabetic rats. Rev. Bras. Farmacogn. 2018, 28, 716–723. [Google Scholar] [CrossRef]
- Cerletti, P.; Duranti, M. Development of lupine proteins. J. Am. Oil Chem. Soc. 1979, 56, 460–463. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Bryant, L.; Rangan, A.; Grafenauer, S. Lupins and Health Outcomes: A Systematic Literature Review. Nutrients 2022, 14, 327. [Google Scholar] [CrossRef]
- Heinzl, G.C.; Tretola, M.; De Benedetti, S.; Silacci, P.; Scarafoni, A. γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients 2022, 14, 3666. [Google Scholar] [CrossRef] [PubMed]
- García López, P.M.; de la Mora, P.G.; Wysocka, W.; Maiztegui, B.; Alzugaray, M.E.; Del Zotto, H.; Borelli, M.I. Quinolizidine alkaloids isolated from Lupinus species enhance insulin secretion. Eur. J. Pharmacol. 2004, 504, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Díaz, C.; Borelli, M.; Przybyl, A.; Garcia-Lopez, J.; Garzon, D.L.M.; García-López, P. Insulin secretion effect of 2, 17-dioxosparteine, 17-thionosparteine, multiflorine and 17-hydroxy-lupanine on rat Langerhan’s islets. In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Australia, 14–18 September 2008; International Lupin Association: Canterbury, New Zealand, 2008; pp. 484–487. [Google Scholar]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Luzi, L.; Pozza, G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol. 1997, 34, 239–244. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Batiha, G.E.-S.; Ogunlana, O.O.; Chinedu, S.N. Terminalia catappa Extract Palliates Redox Imbalance and Inflammation in Diabetic Rats by Upregulating Nrf-2 Gene. Int. J. Inflam. 2021, 2021, 9778486. [Google Scholar] [CrossRef]
- Arun, G.; Rajaram, R.; Kaleshkumar, K.; Gayathri, N.; Sivasudha, T.; Kandasamy, S. Synergistic effect of novel chitosan combined metformin drug on streptozotocin-induced diabetes mellitus rat. Int. J. Biol. Macromol. 2020, 153, 1335–1349. [Google Scholar] [CrossRef]
- Knecht, K.T.; Sanchez, P.; Kinder, D.H. Lupine Seeds (Lupinus spp.): History of Use, Use as An Antihyperglycemic Medicinal, and Use as a Food Plant. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 393–402. [Google Scholar]
- Matteucci, E.; Giampietro, O. Proposal open for discussion: Defining agreed diagnostic procedures in experimental diabetes research. J. Ethnopharmacol. 2008, 115, 163–172. [Google Scholar] [CrossRef]
- Saad, M.I.; Kamel, M.A.; Hanafi, M.Y. Modulation of Adipocytokines Production and Serum NEFA Level by Metformin, Glimepiride, and Sitagliptin in HFD/STZ Diabetic Rats. Biochem. Res. Int. 2015, 2015, 138134. [Google Scholar] [CrossRef]
- Mishra, C.; Khalid, M.A.; Fatima, N.; Singh, B.; Tripathi, D.; Waseem, M.; Mahdi, A.A. Effects of citral on oxidative stress and hepatic key enzymes of glucose metabolism in streptozotocin/high-fat-diet induced diabetic dyslipidemic rats. Iran. J. Basic Med. Sci. 2019, 22, 49–57. [Google Scholar] [CrossRef]
- Soto-Luna, I.C.; García-López, P.M.; Vargas-Guerrero, B.; Guzmán, T.J.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupin protein isolate improves insulin sensitivity and steatohepatitis in vivo and modulates the expression of the Fasn, Gys2, and Gsk3b genes. Food Sci. Nutr. 2021, 9, 2549–2560. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Xu, Y.; Hu, X.; Zhang, C.; Pang, S. A Possible Mechanism of Metformin in Improving Insulin Resistance in Diabetic Rat Models. Int. J. Endocrinol. 2019, 2019, 3248527. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep. 2021, 11, 20677. [Google Scholar] [CrossRef] [PubMed]
- Surbala, L.; Singh, C.B.; Devi, R.V.; Singh, O.J. Rutaecarpine exhibits anti-diabetic potential in high fat diet-multiple low dose streptozotocin induced type 2 diabetic mice and in vitro by modulating hepatic glucose homeostasis. J. Pharmacol. Sci. 2020, 143, 307–314. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, T.J.; Düfer, M.; Wiedemann, M.; Olguín-Alor, R.; Soldevila, G.; Gurrola-Díaz, C.M. Lupin γ-conglutin protects against cell death induced by oxidative stress and lipotoxicity, but transiently inhibits in vitro insulin secretion by increasing KATP channel currents. Int. J. Biol. Macromol. 2021, 187, 76–90. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; Grobbee, D.E.; van der Schouw, Y.T. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010, 33, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Illesca, P.G.; Álvarez, S.M.; Selenscig, D.A.; Ferreira, M.D.R.; Giménez, M.S.; Lombardo, Y.B.; D’Alessandro, M.E. Dietary soy protein improves adipose tissue dysfunction by modulating parameters related with oxidative stress in dyslipidemic insulin-resistant rats. Biomed. Pharmacother. 2017, 88, 1008–1015. [Google Scholar] [CrossRef]
- Huang, Y.; Ashaolu, T.J.; Olatunji, O.J. Micronized Dietary Okara Fiber: Characterization, Antioxidant, Antihyperglycemic, Antihyperlipidemic, and Pancreato-Protective Effects in High Fat Diet/Streptozotocin-Induced Diabetes Mellitus. ACS Omega 2022, 7, 19764–19774. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, D.; Oceguera-Contreras, E.; Salazar-Montes, A.; González-Cuevas, J.; Hernández-Ortega, L.D.; Armendáriz-Borunda, J. Nrf2 and Snail-1 in the prevention of experimental liver fibrosis by caffeine. World J. Gastroenterol. 2013, 19, 9020–9033. [Google Scholar] [CrossRef]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The influence of alkaloids on oxidative stress and related signaling pathways. Free. Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef]
- Navik, U.; Sheth, V.G.; Kabeer, S.W.; Tikoo, K. Dietary Supplementation of Methyl Donor l-Methionine Alters Epigenetic Modification in Type 2 Diabetes. Mol. Nutr. Food Res. 2019, 63, e1801401. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Garcia-Martin, R.; Cai, W.; Konishi, M.; O’Neill, B.T.; Sakaguchi, M.; Kim, J.H.; Jung, D.Y.; Kim, J.K.; Kahn, C.R. Multi-dimensional Transcriptional Remodeling by Physiological Insulin In Vivo. Cell Rep. 2019, 26, 3429–3443.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Ming, Y.N.; Zhang, J.Y.; Chen, X.Y.; Zeng, M.D.; Mao, Y.M. Gene-metabolite network analysis in different nonalcoholic fatty liver disease phenotypes. Exp. Mol. Med. 2017, 49, e283. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Wang, G.; Yang, Z. Ligustilide alleviates the insulin resistance, lipid accumulation, and pathological injury with elevated phosphorylated AMPK level in rats with diabetes mellitus. J. Recept. Signal Transduct. 2021, 41, 85–92. [Google Scholar] [CrossRef]
- Kubota, N.; Tobe, K.; Terauchi, Y.; Eto, K.; Yamauchi, T.; Suzuki, R.; Tsubamoto, Y.; Komeda, K.; Nakano, R.; Miki, H.; et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 2000, 49, 1880–1889. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.J. IRS2 takes center stage in the development of type 2 diabetes. J. Clin. Investig. 2004, 114, 886–888. [Google Scholar] [CrossRef]
- Wang, L.; Duan, J.; Jia, N.; Liu, M.; Cao, S.; Weng, Y.; Zhang, W.; Cao, J.; Li, R.; Cui, J.; et al. IRS-2/Akt/GSK-3β/Nrf2 Pathway Contributes to the Protective Effects of Chikusetsu Saponin IVa against Lipotoxicity. Oxidative Med. Cell. Longev. 2021, 2021, 8832318. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidine alkaloids. In Methods in Plant Biochemistry; Academic Press: London, UK, 1993; pp. 197–239. [Google Scholar]
- Oeh, R.; Rieblinger, K.; Wink, M. Process for Extracting Alkaloids, in Particular Lupanin, from Alkaloid-Containing Plants. WO 19995032968 or DE 4418618 C1, 24 August 1995. [Google Scholar]
- Wysocka, W.; Przybył, A.; Brukwicki, T. The structure of angustifoline, an alkaloid ofLupinus angustifolius, in solution. Mon. Chem. 1994, 125, 1267–1272. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, S.; Wu, J.; Luo, L.; Qiao, S.; Li, R.; Xu, W.; Wang, N.; Zhao, B.; Wang, X.; et al. A specific gut microbiota and metabolomic profiles shifts related to antidiabetic action: The similar and complementary antidiabetic properties of type 3 resistant starch from Canna edulis and metformin. Pharmacol. Res. 2020, 159, 104985. [Google Scholar] [CrossRef]
- Cheng, K.C.; Li, Y.; Cheng, J. The Areas Under Curves (AUC) used in diabetes research: Update view. Int. Obes. Diabetes 2015, 4, 1–2. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Tiessen, A.; Soto-Luna, I.C.; Peniche-Pavía, H.A.; Vargas-Guerrero, B.; Domínguez-Rosales, J.A.; García-López, P.M.; Gurrola-Díaz, C.M. Consumption of nixtamal from a new variety of hybrid blue maize ameliorates liver oxidative stress and inflammation in a high-fat diet rat model. J. Funct. Foods 2020, 72, 104075. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]






| Experimental Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Control | T2D Control | T2D Cγ 28 + Lupanine 20 | T2D Metformin 300 + Glibenclamide 10 | |||||
| Pre Treatment | Post Treatment | Pre Treatment | Post Treatment | Pre Treatment | Post Treatment | Pre Treatment | Post Treatment | |
| Glucose (mg/dL) | 112.6 ± 5.1 | 114.6 ± 7.4 | 479.2 ± 14.0 | 482.2 ± 26.2 | 564.6 ± 29.4 | 352.4 ± 54.7 ** | 299.0 ± 50.9 | 227.0 ± 19.2 |
| Triglycerides (mg/dL) | 50.2 ± 5.7 | 50.4 ± 2.9 | 86.0 ± 9.1 | 55.8 ± 12.4 | 283.4 ± 35.5 | 75.6 ± 13.5 *** | 104.2 ± 29.4 | 53.8 ± 12.5 |
| T. Cholesterol (mg/dL) | 50.8 ± 5.0 | 56.4 ± 2.8 | 142.0 ± 7.9 | 161.0 ± 16.2 | 276.8 ± 76.2 | 189.6 ± 39.6 | 166.6 ± 14.9 | 110.6 ± 6.4 ** |
| HDL-c (mg/dL) | 36.6 ± 5.0 | 42.6 ± 1.9 | 49.8 ± 11.0 | 56.0 ± 13.9 | 23.8 ± 2.0 | 33.2 ± 4.2 | 26.2 ± 2.2 | 30.0 ± 1.2 |
| LDL-c (mg/dL) | 7.5 ± 3.0 | 7.8 ± 2.8 | 104.6 ± 3.6 | 131.8 ± 9.3 | 238.8 ± 68.0 | 162.0 ± 31.5 | 121.4 ± 3.2 | 79.2 ± 3.3 * |
| AST (U/L) | 57.6 ± 4.9 | 52.0 ± 2.8 | 248.6 ± 77.6 | 367.8 ± 76.0 | 144.2 ± 19.1 | 294.4 ± 31.9 * | 155.0 ± 4.2 | 192.2 ± 20.6 |
| ALT (U/L) | 33.6 ± 3.2 | 28.8 ± 2.2 | 249.4 ± 103.2 | 430.4 ± 111.5 | 104.0 ± 10.3 | 231.8 ± 71.6 | 61.2 ± 4.8 | 121.2 ± 26.6 |
| Urea (mg/dL) | 37.6 ± 5.3 | 36.6 ± 0.9 | 57.2 ± 5.9 | 59.4 ± 4.9 | 61.6 ± 3.1 | 57.0 ± 4.2 | 37.0 ± 2.9 | 49.2 ± 4.2 * |
| Creatinine (mg/dL) | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.2 | 0.6 ± 0.0 ** | 1.3 ± 0.3 | 0.7 ± 0.1 ** | 0.7 ± 0.0 | 0.7 ± 0.0 |
| T2D Control and T2D Treated with Cγ + Lupanine | |||
|---|---|---|---|
| Upregulated | Downregulated | ||
| Gene | Fold Change | Gene | Fold Change |
| Chac1 | 6.43 | Irg1 | −3.20 |
| Coq10b | 6.14 | Inmt | −2.97 |
| Cyp2b1 | 5.28 | Ly6c | −2.97 |
| Insig1 | 4.33 | Ly6c | −2.87 |
| Cyp2b2 | 3.75 | Ly6c | −2.84 |
| Tsku | 3.39 | Snai2 | −2.60 |
| PVR | 3.32 | Rtp3 | −2.18 |
| Slc38a2 | 3.25 | Lcn12 | −2.15 |
| Btg2 | 3.15 | Evi2b | −2.14 |
| Slc20a1 | 3.09 | Nr1i3 | −2.12 |
| Restored Genes | Restored > 50% | |||
|---|---|---|---|---|
| Alpl | Ifrd1 | Alas1 | Gstk1 | Rgs4 |
| Btg2 | Jun | Angptl4 | Gzmbl3 | Rictor |
| Chka | Kcnk5 | Bcl2l11 | Hao1 | Serpine1 |
| Coq10b | Lcn12 | Bmf | Igfbp1 | Slc19a2 |
| Cyp2b1 | Map2k6 | C8g | Inmt | Slc1a4 |
| Cyp2b2 | Nrip1 | Casp12 | Irs2 | Slc20a1 |
| Ddit3 | Pdha1 | Casp2 | Kcnj11 | Slc37a4 |
| Dld | Pdk4 | Ces2c | Kcnj14 | Slc38a2 |
| Dusp8 | Rtp3 | Esr1 | Klf6 | Tat |
| F12 | Slc25a25 | Fgf21 | Myc | Thap1 |
| Gadd45b | Slc3a2 | Fgf23 | Pcyt1a | Thap2 |
| Gcgr | Snai2 | Foxo1 | Pdha1l1 | Thap3 |
| Gdf15 | Tsku | Foxo3 | Ppargc1a | Tp53inp1 |
| Gem | G6pc | Rell1 | Tp53inp2 | |
| Gpcpd1 | Rgs1 | Zfp354a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Ávila, P.L.; Guzmán, T.J.; Domínguez-Rosales, J.A.; García-López, P.M.; Cervantes-Garduño, A.B.; Wink, M.; Gurrola-Díaz, C.M. Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile. Pharmaceuticals 2023, 16, 117. https://doi.org/10.3390/ph16010117
Guerra-Ávila PL, Guzmán TJ, Domínguez-Rosales JA, García-López PM, Cervantes-Garduño AB, Wink M, Gurrola-Díaz CM. Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile. Pharmaceuticals. 2023; 16(1):117. https://doi.org/10.3390/ph16010117
Chicago/Turabian StyleGuerra-Ávila, Paloma Lucía, Tereso J. Guzmán, José Alfredo Domínguez-Rosales, Pedro Macedonio García-López, Alejandra Beatriz Cervantes-Garduño, Michael Wink, and Carmen Magdalena Gurrola-Díaz. 2023. "Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile" Pharmaceuticals 16, no. 1: 117. https://doi.org/10.3390/ph16010117
APA StyleGuerra-Ávila, P. L., Guzmán, T. J., Domínguez-Rosales, J. A., García-López, P. M., Cervantes-Garduño, A. B., Wink, M., & Gurrola-Díaz, C. M. (2023). Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile. Pharmaceuticals, 16(1), 117. https://doi.org/10.3390/ph16010117