Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity and Phenolic Content of CJL Extracts
2.2. Identification of CJL3 Components
2.3. Suppression of Nitric Oxide (NO) and Pro-Inflammatory Cytokine Production by CJL3
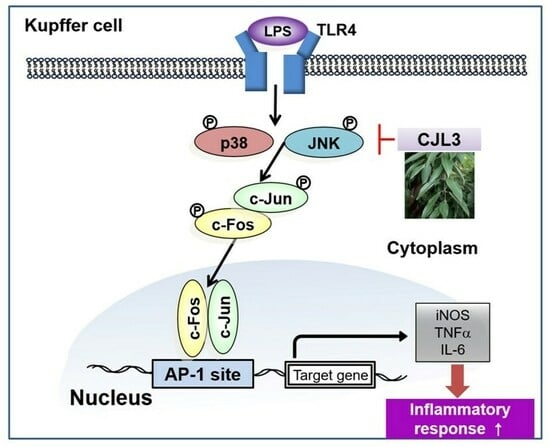
2.4. Inhibition of the LPS-Induced p38/Jun N-Terminal Kinase (JNK)/AP-1 Signaling Pathway by CJL3
3. Discussion
4. Materials and Methods
4.1. Chemical Extracts of Cinnamomum japonicum Sieb. Leaf
4.2. Antioxidative Activities and Phenolic Content
4.2.1. DPPH Radical-Scavenging Activity
4.2.2. ABTS Radical-Scavenging Activity
4.2.3. TPC and TFC
4.3. High-Performance Liquid Chromatography with Diode Array Detection (HPLC–DAD) Analysis
4.4. Quantitative Analysis of Polyphenols Using HPLC MS/MS
4.5. Cell Culture
4.6. MTT Assays
4.7. Analysis of Nitric Oxide Production
4.8. Immunoblot Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. RT-PCR Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Bansal, M.B. Role of Kupffer cells in driving hepatic inflammation and fibrosis in HIV infection. Front. Immunol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Drummond, R.A. Kupffer cells mediate systemic antifungal immunity. Trends Immunol. 2019, 40, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Uosef, A.; Kubiak, J.Z.; Ghobrial, R.M. Macrophage proinflammatory responses to microorganisms and transplanted organs. Int. J. Mol. Sci. 2020, 21, 9669. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer cells in non-alcoholic fatty liver disease: Friend or foe? Int. J. Biol. Sci. 2020, 16, 2367. [Google Scholar] [CrossRef] [PubMed]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W. Kupffer cells: Inflammation pathways and cell-cell interactions in alcohol-associated liver disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, B.; Yang, Y.; Ferguson, D.K. Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol. 2022, 12, e9378. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef] [PubMed]
- Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Heyat, M.B.B.; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on bioactive molecules, antioxidant, anti-inflammatory, and other pharmacological activities of Cinnamomum camphora Linn. Oxidative Med. Cell. Longev. 2022, 2022, 9354555. [Google Scholar] [CrossRef]
- Eweys, A.S.; Zhao, Y.-S.; Darwesh, O.M. Improving the antioxidant and anticancer potential of Cinnamomum cassia via fermentation with Lactobacillus plantarum. Biotechnol. Rep. 2022, 36, e00768. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C.; Mishra, A. Neuroprotective potential of cinnamon and its metabolites in Parkinson’s disease: Mechanistic insights, limitations, and novel therapeutic opportunities. J. Biochem. Mol. Toxicol. 2021, 35, e22720. [Google Scholar] [CrossRef]
- Farazande, M.; Shabab, S.; Mahmoudabady, M.; Gholamnezhad, Z. Effects of Cinnamon on Risk Factors of Cardiovascular Diseases: A Review Paper. Intern. Med. Today 2021, 28, 16–37. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, M.H.; Yang, J.H. Cinnamomum japonicum Siebold Branch Extracts Attenuate NO and ROS Production via the Inhibition of p38 and JNK Phosphorylation. Molecules 2023, 28, 1974. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Shin, H.-J. Antioxidant Activity and Polyphenol Composition of Leaf and Branch of a Warm Temperature Plant, Cinnamomum japonicum Siebold. J. Adv. Eng. Technol. 2020, 13, 123–129. [Google Scholar]
- He, Z.-D.; Qiao, C.-F.; Han, Q.-B.; Cheng, C.-L.; Xu, H.-X.; Jiang, R.-W.; But, P.P.-H.; Shaw, P.-C. Authentication and Quantitative Analysis on the Chemical Profile of Cassia Bark (Cortex cinnamomi) by High-Pressure Liquid Chromatography. J. Agric. Food Chem. 2005, 53, 2424–2428. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Intestinal anti-inflammatory effects of cinnamon extracts in a co-culture model of intestinal epithelial Caco-2 cells and RAW264.7 macrophages. Appl. Biol. Chem. 2017, 60, 553–561. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Ameyar, M.; Wisniewska, M.; Weitzman, J.B. A role for AP-1 in apoptosis: The case for and against. Biochimie 2003, 85, 747–752. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.R.; Tian, Z. Roles of hepatic stellate cells in acute liver failure: From the perspective of inflammation and fibrosis. World J. Hepatol. 2019, 11, 412–420. [Google Scholar] [CrossRef]
- Cha, Y.Y.; Kim, C.T.; Cho, Y.J. Effect of Extraction Methods on Flavoring Compounds and Antioxidant Activity of Extracts from Cinnamon (Cinnamomum cassia Blume). Food Eng. Prog. 2018, 22, 304–308. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G.; Zhang, H.; Xie, H.; Jiang, Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov. Food Sci. Emerg. Technol. 2009, 10, 627–632. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxidative Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Lende, A.B.; Kshirsagar, A.D.; Deshpande, A.D.; Muley, M.M.; Patil, R.R.; Bafna, P.A.; Naik, S.R. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 2011, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- Garces de Los Fayos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Tao, H.; Peng, Y.; Wang, S.; Zhong, Z.; El-Seedi, H.; Dragan, S.; Zengin, G.; San Cheang, W.; Wang, Y. The anti-inflammatory potential of Portulaca oleracea L.(purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019, 290, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Li, W.; Cai, M.; Yan, J.; Zang, C.; Cai, R.; Gao, Y.; Qi, Y. Edible O. fragrans flower ameliorates LPS-induced inflammatory responses through suppressing NF-κB and AP-1 pathways. J. Funct. Foods 2023, 104, 105505. [Google Scholar] [CrossRef]
- Choi, M.-H.; Yang, S.-H.; Shin, H.-J. Inhibition of Melanogenesis by domestic bamboo leaves (Sasa coreana Nakai) extract in B16F10 melanoma cells. KSBB J. 2018, 33, 41–47. [Google Scholar] [CrossRef]
- Choi, M.-H.; Jo, H.-G.; Yang, J.H.; Ki, S.H.; Shin, H.-J. Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 409. [Google Scholar] [CrossRef]
- Lee, S.-O.; Lee, H.-J.; Yu, M.-H.; Im, H.-G.; Lee, I.-S. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ullung island. Korean J. Food Sci. Technol. 2005, 37, 233–240. [Google Scholar]
- Choi, S.-Y.; Kim, Y.-C.; Chang, B.-S. Inhibitory efficacy of black tea water extract on melanogenesis in melan-a cells and its action mechanisms. Appl. Microsc. 2011, 41, 169–177. [Google Scholar]
- Eom, S.H.; Park, H.J.; Jin, C.W.; Kim, D.O.; Seo, D.W.; Jeong, Y.H.; Cho, D.H. Changes in antioxidant activity with temperature and time in Chrysanthemum indicum L.(Gamguk) teas during elution processes in hot water. Food Sci. Biotechnol. 2008, 17, 408–412. [Google Scholar]
- Yang, J.H.; Kim, K.M.; Kim, M.G.; Seo, K.H.; Han, J.Y.; Ka, S.O.; Park, B.H.; Shin, S.M.; Ku, S.K.; Cho, I.J.; et al. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic. Biol. Med. 2015, 78, 156–167. [Google Scholar] [CrossRef]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef] [PubMed]







| No. | Compound | Retention Time | CJL3 (Unit: μ/g) |
|---|---|---|---|
| 1 | Gallic acid | - | - |
| 2 | Catechin | 14.102 | 237.19 ± 32.57 |
| 3 | Epigallocatechin gallate | 17.522 | 191.84 ± 30.77 |
| 4 | (-)-Epicatechin | 18.631 | 1238.46 ± 275.57 |
| 5 | Ethyl gallate | 22.051 | 145.78 ± 6.17 |
| 6 | Rutin | - | |
| 7 | p-Coumaric acid | 24.981 | 210.94 ± 99.94 |
| 8 | Coumarin | 34.907 | 3.96 ± 4.93 |
| 9 | Cinnamylalcohol | 36.909 | 191.53 ± 7.23 |
| 10 | Quercetin | 40.617 | 88.69 ± 39.45 |
| 11 | Cinnamylaldehyde | 45.263 | 11.14 ± 4.68 |
| 12 | trans-Cinnamic acid | - | - |
| 13 | Eugenol | 51.993 | 196.07 ± 39.43 |
| 14 | Cinnamyl acetate | 63.396 | 5261.47 ± 247.13 |
| Gene | Sequence (5′-3′) | |
|---|---|---|
| mouse iNOS | sense | 5′-CCTCCTCCACCCTACCAAGT-3′ |
| antisense | 5′-CACCCAAAGTGCTTCAGTCA-3′ | |
| mouse TNF-α | sense | 5′-AAGCCTGTAGCCCACGTCGTA-3′ |
| antisense | 5′-AGGTACAACCCATCGGCTGG-3′ | |
| mouse IL-6 | sense | 5′-TCCATCCAGTTGCCTTCTTG-3′ |
| antisense | 5′-TTCCACGATTTCCCAGAGAAC-3′ | |
| mouse GAPDH | sense | 5′-TGCCCCCATGTTTGTGATG-3′ |
| antisense | 5′-TGTGGTCATGAGCCCTTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Jung, I.A.; Kim, J.M.; Choi, M.-H.; Yang, J.H. Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling. Pharmaceuticals 2023, 16, 1402. https://doi.org/10.3390/ph16101402
Kim JM, Jung IA, Kim JM, Choi M-H, Yang JH. Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling. Pharmaceuticals. 2023; 16(10):1402. https://doi.org/10.3390/ph16101402
Chicago/Turabian StyleKim, Ji Min, In A Jung, Jae Min Kim, Moon-Hee Choi, and Ji Hye Yang. 2023. "Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling" Pharmaceuticals 16, no. 10: 1402. https://doi.org/10.3390/ph16101402
APA StyleKim, J. M., Jung, I. A., Kim, J. M., Choi, M. -H., & Yang, J. H. (2023). Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling. Pharmaceuticals, 16(10), 1402. https://doi.org/10.3390/ph16101402