Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant Identifications
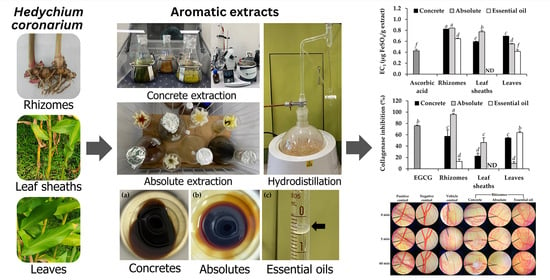
2.2. Aromatic Extracts from H. coronarium
2.3. Chemical Compositions of H. coronarium
2.4. Antioxidant Activities of H. coronarium Aromatic Extracts
2.5. Anti-Skin Wrinkle Activities of H. coronarium Aromatic Extracts
2.6. Irritation Potency of H. coronarium Aromatic Extracts
3. Materials and Methods
3.1. Plant Materials and Identifications
3.2. Chemical Materials
3.3. Plant Extraction
3.3.1. Concrete
3.3.2. Absolute
3.3.3. Essential Oil
3.4. Determination of Chemical Compositions via Gas Chromatography–Mass Spectrometry (GC-MS)
3.5. Antioxidant Activities Determination
3.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.5.2. 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-sulfonic acid) (ABTS) Assay
3.5.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.6. Anti-Skin Aging Activities Determination
3.6.1. Collagenase Inhibitory Activities Determination
3.6.2. Elastase Inhibitory Activities Determination
3.6.3. Hyaluronidase Inhibitory Activities Determination
3.7. Hen’s Egg–Chorioallantoic Membrane (HET-CAM) Test
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, N.K.; Nguyen, H.T.; Nguyen, Q.B. A review on the ethnomedicinal uses, phytochemistry and pharmacology of plant species belonging to Kaempferia L. genus (Zingiberaceae). Pharm. Sci. Asia. 2021, 48, 1–24. [Google Scholar] [CrossRef]
- Voon, K.J.; Sivasothy, Y.; Sundralingam, U.; Lalmahomed, A.; Goh, A.P. Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. J. Pharm. 2022, 15, 1517. [Google Scholar]
- Rachkeeree, A.; Kantadoung, K.; Suksathan, R.; Puangpradab, R.; Page, P.A.; Sommano, S.R. Nutritional Compositions and Phytochemical Properties of the Edible Flowers from Selected Zingiberaceae Found in Thailand. Front. Nutr. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Fan, Y. Auxin-responsive R2R3-MYB transcription factors HcMYB1 and HcMYB2 activate volatile biosynthesis in Hedychium coronarium flowers. Front. Plant Sci. 2021, 12, 710826. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Jena, S.; Kar, B.; Sahoo, A.; Panda, P.C.; Nayak, S.; Mahapatra, N. Volatile metabolite profiling of ten Hedychium species by gas chromatography mass spectrometry coupled to chemometrics. Ind. Crops Prod. 2018, 126, 135–142. [Google Scholar] [CrossRef]
- Tavares, W.R.; Barreto, M.D.C.; Seca, A.M. Uncharted source of medicinal products: The case of the Hedychium genus. J. Med. 2020, 7, 23. [Google Scholar] [CrossRef]
- Tian, M.; Wu, X.; Lu, T.; Zhao, X.; Wei, F.; Deng, G.; Zhou, Y. Phytochemical analysis, antioxidant, antibacterial, cytotoxic, and enzyme inhibitory activities of Hedychium flavum rhizome. Front. Pharmacol. 2020, 11, 572659. [Google Scholar] [CrossRef]
- Kamble, K.G.; Dale, A.V. A review on pharmacognostic and pharmacological approach of different species of Hedychium. Indo Am. J. Pharm. Sci. 2018, 5, 6030–6036. [Google Scholar] [CrossRef]
- Lima, A.S.; Junior, H.N.P.C.; Costa-Junior, L.M.; Monteiro, O.S.; Maia, J.G.S.; da Rocha, C.Q. Anthelmintic effect of essential rhizome oil from Hedychium coronarium Koenig (Zingiberaceae) introduced in Northeastern Brazil. Acta Trop. 2021, 218, 105912. [Google Scholar] [CrossRef]
- da Silva, C.F.; Petró, R.R.; Almeida, R.N.; Cassel, E.; Vargas, R.M. On the production and release of Hedychium coronarium essential oil from nanoformulations. Ind. Crops Prod. 2021, 171, 113984. [Google Scholar] [CrossRef]
- Das, R.; Nayak, R.K. Ethnomedicinal uses, phytochemical analysis and antibacterial activity of Hedychium coronarium J. Koenig rhizome. Int. J. Herb. Med. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Peng, W.; Li, P.; Ling, R.; Wang, Z.; Feng, X.; Liu, J.; Yan, J. Diversity of volatile compounds in ten varieties of Zingiberaceae. Molecules. 2022, 27, 565. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.S.; Roosta, A. The effect of different solvents on the production of rose concrete and rose absolute, experimental study and thermodynamic aspects using the UNIFAC model. Chem. Eng. Res. Des. 2022, 184, 326–337. [Google Scholar] [CrossRef]
- Behera, S.; Rath, S.; Akhtar, M.S.; Naik, S.K. Biotechnological intervention through tissue culture in Hedychium coronarium: A potential anticancer plant. In Anticancer Plants: Natural Products and Biotechnological Implements: Volume 2; Springer: Singapore, 2018; pp. 551–564. [Google Scholar] [CrossRef]
- Sarangthem, N.; Talukdar, N.C.; Thongam, B. Collection and evaluation of Hedychium species of Manipur, Northeast India. Genet. Resour. Crop Evol. 2013, 60, 13–21. [Google Scholar] [CrossRef]
- Arya, S.; Kumar, R.; Prakash, O.; Rawat, A.; Mahawer, S.K.; Rawat, D.S.; de Oliveira, M. Hedychium coronarium J. Koenig: Traditional uses, phytochemistry, biological activities and future aspects. Curr. Org. Chem. 2022, 26, 1676–1690. [Google Scholar] [CrossRef]
- Shanmugam, P.V.; Yadav, A.; Chanotiya, C.S. Enantiomer differentiation of key volatile constituents from leaves, stems, rhizome and flowers of cultivated Hedychium coronarium Koenig from India. J. Essent. Oil Res. 2015, 27, 101–106. [Google Scholar] [CrossRef]
- Baydar, H.; Kineci, S. Scent composition of essential oil, concrete, absolute and hydrosol from lavandin (Lavandula x intermedia Emeric ex Loisel. J. Essent. Oil-Bear. Plants 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Parida, R.; Mohanty, S.; Nayak, S. Chemical composition of essential oil from leaf and rhizome of micropropagated and conventionally grown Hedychium coronarium Koen. from Eastern India. J. Essent. Oil-Bear. Plants 2015, 18, 161–167. [Google Scholar] [CrossRef]
- dos Santos, B.C.; Barata, L.E.; Marques, F.A.; Baroni, A.C.; Karnos, B.A.; de Oliveira, P.R.; Guerrero, P.G., Jr. Composition of leaf and rhizome essential oils of Hedychium coronarium Koen. from Brazil. J. Essent. Oil Res. 2010, 22, 305–306. [Google Scholar] [CrossRef]
- Fu, C.; Li, Z.; Jia, C.; Zhang, W.; Zhang, Y.; Yi, C.; Xie, S. Recent advances on bio-based isobutanol separation. Energy Convers. Manag. X 2021, 10, 100059. [Google Scholar] [CrossRef]
- Tan, F.; Li, Y.; Xie, Z.; Bian, X.; Du, F.; Liu, S.; Lu, P.; Wang, J. Geochemical Characteristics of the Jurassic Alkane Gas in the Muli Depression, South Qilian Basin: Implications for Potential of Light Oil and Condensate. Front. Environ. Sci. 2022, 10, 898629. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tunnisa, F.; Faridah, D.N.; Afriyanti, A.; Rosalina, D.; Syabana, M.A.; Darmawan, N.; Yuliana, N.D. Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 2022, 14, 100285. [Google Scholar] [CrossRef] [PubMed]
- Rocha Caldas, G.F.; Oliveira, A.R.D.S.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; Silva-Neto, J.D.C.; Costa-Silva, J.H.C.; Ferreira, F.; da Costa, J.G.M.; Wanderley, A.G. Gastroprotective mechanisms of the monoterpene 1, 8-cineole (eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Wiśniewska, R. Interactions of Selected Monoterpenes with Iron and Copper Ions Based on Ferrozine and CUPRAC Methods–the Preliminary Studies. Chem. Biodivers. 2022, 19, e202200461. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Aksoy, L.; Kolay, E.; Ağılönü, Y.; Aslan, Z.; Kargıoğlu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2017, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The pathobiology of skin aging: New insights into an old dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, T.; Salman, S. Hyaluronic Acid Dermal Fillers. Neurotoxins Fill. Facial Esthet. Surg. 2019, 63–69. [Google Scholar] [CrossRef]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Singh, M.; McKenzie, K.; Ma, X. Effect of dimethyl sulfoxide on in vitro proliferation of skin fibroblast cells. J. Biotech Res. 2017, 8, 78. [Google Scholar]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Oyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. Ther. 2011, 5, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and mediterranean region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Steiling, W.; Bracher, M.; Courtellemont, P.; De Silva, O. The HET–CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. In Vitro 1999, 13, 375–384. [Google Scholar] [CrossRef]







| RT (min) | Chemical Components | MW | Formula | Rhizomes | Leaf Sheaths | Leaves | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | AB | EO | CC | AB | CC | AB | EO | ||||
| 4.01 | 3-Methyl-3-pentanethiol | 118.2 | C6H14S | 0.2 ± 0.0 | - | - | 3.1 ± 0.5 | - | 0.6 ± 0.2 | - | - |
| 4.14 | α-Thujene | 136.2 | C10H16 | 0.1 ± 0.0 | - | 0.2 ± 0.0 | - | - | 0.2 ± 0.0 | - | 0.2 ± 0.0 |
| 4.31 | α-Pinene | 136.2 | C10H16 | 3.7 ± 0.0 | - | 7.6 ± 0.6 | 9.1 ± 0.1 | - | 6.2 ± 0.0 | 0.3 ± 0.0 | 10.8 ± 0.2 |
| 4.64 | Camphene | 136.2 | C10H16 | 0.3 ± 0.0 | - | 0.8 ± 0.1 | 0.7 ± 0.1 | - | 0.2 ± 0.0 | - | 0.2 ± 0.0 |
| 4.82 | Benzaldehyde | 106.1 | C7H6O | 1.2 ± 0.0 | - | - | 15.0 ± 3.4 | - | 2.5 ± 1.1 | - | - |
| 5.05 | β-Phellandrene | 136.2 | C10H16 | - | - | 0.1 ± 0.0 | - | - | 1.1 ± 0.0 | - | 3.6 ± 0.3 |
| 5.20 | β-Pinene | 136.2 | C10H16 | 12.0 ± 0.1 | 1.2 ± 0.1 | 19.8 ± 0.6 | 20.1 ± 1.3 | - | 21.3 ± 0.7 | 0.9 ± 0.1 | 31.4 ± 0.0 |
| 5.34 | β-Myrcene | 136.2 | C10H16 | 0.3 ± 0.0 | - | 0.3 ± 0.1 | - | - | - | - | 0.6 ± 0.0 |
| 5.81 | α-Phellandrene | 136.2 | C10H16 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.6 ± 0.1 | - | - | 0.3 ± 0.1 | - | 0.1 ± 0.0 |
| 6.07 | Terpinene | 136.2 | C10H17 | 0.2 ± 0.0 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.0 | 2.2 ± 0.4 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| 6.39 | D-Limonene | 136.2 | C10H16 | 1.0 ± 0.0 | 1.4 ± 0.1 | - | 5.2 ± 0.1 | 0.1 ± 0.1 | 1.2 ± 0.1 | 0.2 ± 0.0 | 5.9 ± 0.0 |
| 6.49 | Eucalyptol | 154.3 | C10H18O | 67.8 ± 1.0 | 72.9 ± 1.9 | 53.6 ± 0.5 | 30.1 ± 4.4 | 14.0 ± 1.2 | 19.6 ± 6.0 | 1.0 ± 0.2 | 18.4 ± 0.4 |
| 8.37 | Linalool | 154.3 | C10H18O | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.1 | - | - | 0.1 ± 0.1 | - | 0.1 ± 0.0 |
| 10.91 | Borneol | 154.3 | C10H18O | 0.5 ± 0.0 | 0.8 ± 0.2 | 0.6 ± 0.1 | 1.2 ± 0.0 | 5.4 ± 0.1 | 0.4 ± 0.0 | - | 0.3 ± 0.0 |
| 11.22 | Terpinen-4-ol | 154.3 | C10H18O | 2.5 ± 0.1 | 3.5 ± 0.2 | 3.5 ± 0.4 | 0.7 ± 0.0 | 3.7 ± 0.3 | 1.1 ± 0.0 | 0.3 ± 0.0 | 2.7 ± 0.1 |
| 11.74 | L-α-Terpineol | 154.3 | C10H18O | 6.2 ± 0.4 | 12.5 ± 2.0 | 6.2 ± 0.5 | 2.6 ± 0.0 | 22.8 ± 0.7 | 2.4 ± 0.1 | 0.8 ± 0.1 | 2.6 ± 0.1 |
| 17.67 | α-Terpinyl acetate | 196.3 | C12H20O2 | 0.2 ± 0.0 | 0.9 ± 0.0 | 0.1 ± 0.0 | - | 3.3 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 20.60 | β-Caryophyllene | 204.4 | C15H24 | 0.2 ± 0.0 | 1.4 ± 0.1 | 0.1 ± 0.0 | 1.1 ± 0.1 | 27.2 ± 1.3 | 33.7 ± 4.4 | 84.2 ± 0.7 | 9.1 ± 0.5 |
| 22.00 | Humulene | 205.4 | C15H25 | - | - | - | - | 2.4 ± 0.3 | 1.3 ± 0.1 | 6.1 ± 0.1 | 0.5 ± 0.0 |
| 26.86 | Caryophyllene oxide | 220.4 | C15H24O | - | - | - | - | 6.8 ± 0.9 | 1.0 ± 0.3 | 3.8 ± 0.6 | 1.6 ± 0.2 |
| Total | 97.3 | 96.2 | 94.7 | 89.6 | 88.0 | 93.7 | 98.0 | 88.7 | |||
| Samples | Irritation Score | Classification | |
|---|---|---|---|
| Positive control (1% w/v SLS) | 11.6 ± 0.0 a | Severe irritation | |
| Negative control (0.9% w/v NaCl) | 0.0 ± 0.0 c | No irritation | |
| Vehicle control (10% v/v DMSO in olive oil) | 4.8 ± 0.0 b | Mild irritation | |
| Rhizomes | Concrete | 5.8 ± 1.4 b | Moderate irritation |
| Absolute | 4.6 ± 0.1 b | Mild irritation | |
| Essential oil | 5.8 ± 1.4 b | Moderate irritation | |
| Leaf sheaths | Concrete | 0.0 ± 0.0 c | No irritation |
| Absolute | 4.8 ± 0.0 b | Mild irritation | |
| Leaves | Concrete | 4.1 ± 0.1 b | Mild irritation |
| Absolute | 4.7 ± 0.1 b | Mild irritation | |
| Essential oil | 7.3 ± 3.6 b | Moderate irritation | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tammasorn, P.; Charoensup, W.; Bunrod, A.; Kanjanakawinkul, W.; Chaiyana, W. Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition. Pharmaceuticals 2023, 16, 1738. https://doi.org/10.3390/ph16121738
Tammasorn P, Charoensup W, Bunrod A, Kanjanakawinkul W, Chaiyana W. Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition. Pharmaceuticals. 2023; 16(12):1738. https://doi.org/10.3390/ph16121738
Chicago/Turabian StyleTammasorn, Pattiya, Wannaree Charoensup, Anurak Bunrod, Watchara Kanjanakawinkul, and Wantida Chaiyana. 2023. "Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition" Pharmaceuticals 16, no. 12: 1738. https://doi.org/10.3390/ph16121738
APA StyleTammasorn, P., Charoensup, W., Bunrod, A., Kanjanakawinkul, W., & Chaiyana, W. (2023). Promising Anti-Wrinkle Applications of Aromatic Extracts of Hedychium coronarium J. Koenig via Antioxidation and Collagenase Inhibition. Pharmaceuticals, 16(12), 1738. https://doi.org/10.3390/ph16121738