Manipulation of New Fluorescent Magnetic Nanoparticles with an Electromagnetic Needle, Allowed Determining the Viscosity of the Cytoplasm of M-HeLa Cells
Abstract
:1. Introduction
2. Results
2.1. Force Calibration of the Electromagnetic Needle
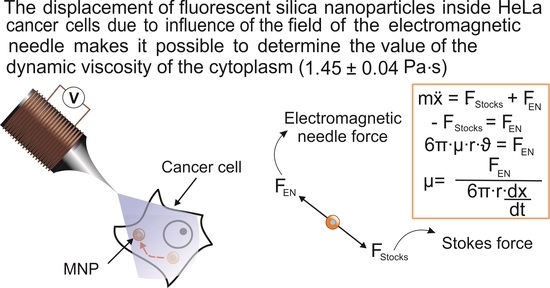
2.2. Effect of the Magnetic Field Gradient on MNPs Loaded into HeLa Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Magnetic Nanoparticles
4.2. Cells
4.3. Electromagnetic Needle
4.4. Calculation of the EN’s Force
4.5. Calculation of the Cytoplasmic Viscosity of HeLa Cancer Cells
4.6. Microscopic Technique
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, J.; Cartmell, S.H.; Keramane, A.; El Haj, A.J. Principles and Design of a Novel Magnetic Force Mechanical Conditioning Bioreactor for Tissue Engineering, Stem Cell Conditioning, and Dynamic in Vitro Screening. IEEE Trans. Nanobiosci. 2006, 5, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel Methodology for Fabrication of Tissue-Engineered Tubular Constructs Using Magnetite Nanoparticles and Magnetic Force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.; Bang, M.; Kim, B.S.; Lee, S.; Kotov, N.A.; Kim, B.; Jeon, D. Intracellular Gold Nanoparticles Increase Neuronal Excitability and Aggravate Seizure Activity in the Mouse Brain. PloS ONE 2014, 9, e91360. [Google Scholar] [CrossRef]
- Schöneborn, H.; Raudzus, F.; Secret, E.; Otten, N.; Michel, A.; Fresnais, J.; Ménager, C.; Siaugue, J.-M.; Zaehres, H.; Dietzel, I.D.; et al. Novel Tools towards Magnetic Guidance of Neurite Growth: (I) Guidance of Magnetic Nanoparticles into Neurite Extensions of Induced Human Neurons and In Vitro Functionalization with RAS Regulating Proteins. J. Funct. Biomater. 2019, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Kunze, A.; Tseng, P.; Godzich, C.; Murray, C.; Caputo, A.; Schweizer, F.E.; Di Carlo, D. Engineering Cortical Neuron Polarity with Nanomagnets on a Chip. ACS Nano 2015, 9, 3664–3676. [Google Scholar] [CrossRef]
- Riggio, C.; Calatayud, M.P.; Giannaccini, M.; Sanz, B.; Torres, T.E.; Fernández-Pacheco, R.; Ripoli, A.; Ibarra, M.R.; Dente, L.; Cuschieri, A.; et al. The Orientation of the Neuronal Growth Process Can Be Directed via Magnetic Nanoparticles under an Applied Magnetic Field. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1549–1558. [Google Scholar] [CrossRef]
- Polte, T.R.; Shen, M.; Karavitis, J.; Montoya, M.; Pendse, J.; Xia, S.; Mazur, E.; Ingber, D.E. Nanostructured Magnetizable Materials That Switch Cells between Life and Death. Biomaterials 2007, 28, 2783–2790. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Zhigachev, A.O.; Klyachko, N.L.; Majouga, A.G.; Sokolsky, M.; Kabanov, A.V. The Dynamics of Magnetic Nanoparticles Exposed to Non-Heating Alternating Magnetic Field in Biochemical Applications: Theoretical Study. J. Nanopart. Res. 2017, 19, 59. [Google Scholar] [CrossRef]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel Method for Rapid Toxicity Screening of Magnetic Nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef]
- Kilinc, D.; Blasiak, A.; O’Mahony, J.J.; Lee, G.U. Low Piconewton Towing of CNS Axons against Diffusing and Surface-Bound Repellents Requires the Inhibition of Motor Protein-Associated Pathways. Sci. Rep. 2015, 4, 7128. [Google Scholar] [CrossRef]
- Pashut, T.; Magidov, D.; Ben-Porat, H.; Wolfus, S.; Friedman, A.; Perel, E.; Lavidor, M.; Bar-Gad, I.; Yeshurun, Y.; Korngreen, A. Patch-Clamp Recordings of Rat Neurons from Acute Brain Slices of the Somatosensory Cortex during Magnetic Stimulation. Front. Cell. Neurosci. 2014, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Dante, S.; Petrelli, A.; Petrini, E.M.; Marotta, R.; Maccione, A.; Alabastri, A.; Quarta, A.; De Donato, F.; Ravasenga, T.; Sathya, A.; et al. Selective Targeting of Neurons with Inorganic Nanoparticles: Revealing the Crucial Role of Nanoparticle Surface Charge. ACS Nano 2017, 11, 6630–6640. [Google Scholar] [CrossRef] [Green Version]
- Shinkai, M.; Yanase, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Intracellular Hyperthermia for Cancer Using Magnetite Cationic Liposomes: In Vitro Study. Jpn. J. Cancer Res. Gann 1996, 87, 1179–1183. [Google Scholar] [CrossRef]
- Long, X.; Ye, J.; Zhao, D.; Zhang, S.-J. Magnetogenetics: Remote Non-Invasive Magnetic Activation of Neuronal Activity with a Magnetoreceptor. Sci. Bull. 2015, 60, 2107–2119. [Google Scholar] [CrossRef] [Green Version]
- Deatsch, A.E.; Evans, B.A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2013, 354, 163–172. [Google Scholar] [CrossRef]
- Dobson, J. Remote Control of Cellular Behaviour with Magnetic Nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef]
- Gualdani, R.; Guerrini, A.; Fantechi, E.; Tadini-Buoninsegni, F.; Moncelli, M.R.; Sangregorio, C. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Modulate HERG Ion Channel Activity. Nanotoxicology 2019, 13, 1197–1209. [Google Scholar] [CrossRef]
- Kargol, A.; Malkinski, L.; Eskandari, R.; Carter, M.; Livingston, D. Cellular Defibrillation: Interaction of Micro-Scale Electric Fields with Voltage-Gated Ion Channels. J. Biol. Phys. 2015, 41, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Mustapić, M.; Ebrahimian, H.; Wagner, P.; Kim, J.H.; Al Hossain, M.S.; Horvat, J.; Martinac, B. Magnetic Nanoparticles for “Smart Liposomes”. Eur. Biophys. J. EBJ 2015, 44, 647–654. [Google Scholar] [CrossRef]
- Mustapić, M.; Al Hossain, M.S.; Horvat, J.; Wagner, P.; Mitchell, D.R.G.; Kim, J.H.; Alici, G.; Nakayama, Y.; Martinac, B. Controlled Delivery of Drugs Adsorbed onto Porous Fe 3 O 4 Structures by Application of AC/DC Magnetic Fields. Microporous Mesoporous Mater. 2016, 226, 243–250. [Google Scholar] [CrossRef]
- Bausch, A.R.; Möller, W.; Sackmann, E. Measurement of Local Viscoelasticity and Forces in Living Cells by Magnetic Tweezers. Biophys. J. 1999, 76, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-Q.; Kuo, C.-Y.; Wei, M.-T.; Wu, K.; Su, P.-T.; Huang, C.-S.; Chiou, A. Intracellular Viscoelasticity of HeLa Cells during Cell Division Studied by Video Particle-Tracking Microrheology. J. Biomed. Opt. 2014, 19, 011008. [Google Scholar] [CrossRef] [PubMed]
- Cenev, Z.; Zhang, H.; Sariola, V.; Rahikkala, A.; Liu, D.; Santos, H.A.; Zhou, Q. Manipulating Superparamagnetic Microparticles with an Electromagnetic Needle. Adv. Mater. Technol. 2018, 3, 1700177. [Google Scholar] [CrossRef] [Green Version]
- Seon, J.A.; Cenev, Z.; Zhou, Q. Automatic Noncontact Extraction and Independent Manipulation of Magnetic Particles Using Electromagnetic Needle. IEEE/ASME Trans. Mechatron. 2020, 25, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Pita-Thomas, W.; Steketee, M.B.; Moysidis, S.N.; Thakor, K.; Hampton, B.; Goldberg, J.L. Promoting Filopodial Elongation in Neurons by Membrane-Bound Magnetic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Goldberg, J.L.; Steketee, M.B. Regulating Growth Cone Motility and Axon Growth by Manipulating Targeted Superparamagnetic Nanoparticles. In Neuromethods; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 135, pp. 89–108. [Google Scholar]
- Fass, J.N.; Odde, D.J. Tensile Force-Dependent Neurite Elicitation via Anti-Β1 Integrin Antibody-Coated Magnetic Beads. Biophys. J. 2003, 85, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Fedorenko, S.; Stepanov, A.; Sibgatullina, G.; Samigullin, D.; Mukhitov, A.; Petrov, K.; Mendes, R.; Rümmeli, M.; Giebeler, L.; Weise, B.; et al. Fluorescent Magnetic Nanoparticles for Modulating the Level of Intracellular Ca 2+ in Motoneurons. Nanoscale 2019, 11, 16103–16113. [Google Scholar] [CrossRef]
- Blokhin, S.D.; Mukhtarov, A.S.; Anikin, A.N.; Samigullin, D.V. Magnetic Stimulator for Influencing Ferromagnetic Nanoparticles in Biomedical Research. Eng. J. Don 2020, 5. Available online: http://www.ivdon.ru/en/magazine/archive/N5y2020/6473 (accessed on 28 January 2023).
- LAojk, J.; Bregar, V.B.; Rajh, M.; Miš, K.; Kreft, M.E.; Pirkmajer, S.; Veranič, P.; Pavlin, M. Cell Type-Specific Response to High Intracellular Loading of Polyacrylic Acid-Coated Magnetic Nanoparticles. Int. J. Nanomed. 2015, 10, 1449–1462. [Google Scholar] [CrossRef] [Green Version]
- Ibragimova, A.R.; Gabdrakhmanov, D.R.; Valeeva, F.G.; Vasileva, L.A.; Sapunova, A.S.; Voloshina, A.D.; Saifina, A.F.; Gubaidullin, A.T.; Danilaev, M.P.; Egorova, S.R.; et al. Mitochondria-Targeted Mesoporous Silica Nanoparticles Noncovalently Modified with Triphenylphosphonium Cation: Physicochemical Characteristics, Cytotoxicity and Intracellular Uptake. Int. J. Pharm. 2021, 604, 120776. [Google Scholar] [CrossRef]
- Bus, T.; Traeger, A.; Schubert, U.S. The Great Escape: How Cationic Polyplexes Overcome the Endosomal Barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Cupic, K.I.; Rennick, J.J.; Johnston, A.P.R.; Such, G.K. Controlling Endosomal Escape Using Nanoparticle Composition: Current Progress and Future Perspectives. Nanomedicine 2019, 14, 215–223. [Google Scholar] [CrossRef]
- Faizullin, B.; Gubaidullin, A.; Gerasimova, T.; Kashnik, I.; Brylev, K.; Kholin, K.; Nizameev, I.; Voloshina, A.; Sibgatullina, G.; Samigullin, D.; et al. “Proton Sponge” Effect and Apoptotic Cell Death Mechanism of Agx-Re6 Nanocrystallites Derived from the Assembly of [{Re6S8}(OH)6–n(H2O)n]n–4 with Ag+ Ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129312. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging. J. Am. Chem. Soc. 2008, 130, 56. [Google Scholar] [CrossRef] [Green Version]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging Intracellular Viscosity of a Single Cell during Photoinduced Cell Death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Berret, J.-F. Local Viscoelasticity of Living Cells Measured by Rotational Magnetic Spectroscopy. Nat. Commun. 2016, 7, 10134. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Minc, N. Cytoskeleton Force Exertion in Bulk Cytoplasm. Front. Cell Dev. Biol. 2020, 8, 69. [Google Scholar] [CrossRef]
- Soloperto, A.; Boccaccio, A.; Contestabile, A.; Moroni, M.; Hallinan, G.I.; Palazzolo, G.; Chad, J.; Deinhardt, K.; Carugo, D.; Difato, F. Mechano-Sensitization of Mammalian Neuronal Networks through Expression of the Bacterial Mechanosensitive MscL Channel. J. Cell Sci. 2018, 131, 210393. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Inositol Trisphosphate and Calcium Signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Steketee, M.B.; Moysidis, S.N.; Jin, X.-L.; Weinstein, J.E.; Pita-Thomas, W.; Raju, H.B.; Iqbal, S.; Goldberg, J.L. Nanoparticle-Mediated Signaling Endosome Localization Regulates Growth Cone Motility and Neurite Growth. Proc. Natl. Acad. Sci. USA 2011, 108, 19042–19047. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Kunze, A.; Murray, C.; Di Carlo, D. Induction of Calcium Influx in Cortical Neural Networks by Nanomagnetic Forces. ACS Nano 2016, 10, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, D.; Qu, Y.; Langer, K.; Öztürk, C.; Zhao, Y.; Chen, C.; Seebohm, G.; Düfer, M.; Fuchs, H.; Galla, H.-J.; et al. Comparison of Cellular Effects of Starch-Coated SPIONs and Poly(Lactic-Co-Glycolic Acid) Matrix Nanoparticles on Human Monocytes. Int. J. Nanomed. 2016, 11, 5221–5236. [Google Scholar] [CrossRef] [PubMed]





| Voltage, V | Current, mA | Needle Magnetic Field Induction, mT | Displacement Value, µm | The Value of the Average Force (at a Distance of 200 μm between the EN and the Particle), pN | The Value of the Average Force (at a Distance of 20 μm between the EN and the Particle), pN |
|---|---|---|---|---|---|
| 1 | 29 | 3.3 ± 0.3 | 1.01 ± 0.03 | 0.056 ± 0.002 | 9.75 ± 1.82 |
| 2 | 59 | 6.0 ± 0.6 | 1.13 ± 0.04 | 0.103 ± 0.017 | 11.47 ± 2.02 |
| 3 | 86 | 7.4 ± 0.6 | 1.23 ± 0.03 | 0.127 ± 0.019 | 14.55 ± 2.61 |
| 4 | 116 | 8.1 ± 0.3 | 1.41 ± 0.05 | 0.198 ± 0.033 | 16.59 ± 2.96 |
| 5 | 144 | 9.5 ± 0.3 | 1.70 ± 0.04 | 0.236 ± 0.050 | 18.30 ± 2.02 |
| 6 | 169 | 10.7 ± 0.3 | 2.03 ± 0.05 | 0.302 ± 0.027 | 37.85 ± 3.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazanova, I.; Suslov, M.; Sibgatullina, G.; Petrov, K.; Fedorenko, S.; Mustafina, A.; Samigullin, D. Manipulation of New Fluorescent Magnetic Nanoparticles with an Electromagnetic Needle, Allowed Determining the Viscosity of the Cytoplasm of M-HeLa Cells. Pharmaceuticals 2023, 16, 200. https://doi.org/10.3390/ph16020200
Ramazanova I, Suslov M, Sibgatullina G, Petrov K, Fedorenko S, Mustafina A, Samigullin D. Manipulation of New Fluorescent Magnetic Nanoparticles with an Electromagnetic Needle, Allowed Determining the Viscosity of the Cytoplasm of M-HeLa Cells. Pharmaceuticals. 2023; 16(2):200. https://doi.org/10.3390/ph16020200
Chicago/Turabian StyleRamazanova, Iliza, Maxim Suslov, Guzel Sibgatullina, Konstantin Petrov, Svetlana Fedorenko, Asiya Mustafina, and Dmitry Samigullin. 2023. "Manipulation of New Fluorescent Magnetic Nanoparticles with an Electromagnetic Needle, Allowed Determining the Viscosity of the Cytoplasm of M-HeLa Cells" Pharmaceuticals 16, no. 2: 200. https://doi.org/10.3390/ph16020200
APA StyleRamazanova, I., Suslov, M., Sibgatullina, G., Petrov, K., Fedorenko, S., Mustafina, A., & Samigullin, D. (2023). Manipulation of New Fluorescent Magnetic Nanoparticles with an Electromagnetic Needle, Allowed Determining the Viscosity of the Cytoplasm of M-HeLa Cells. Pharmaceuticals, 16(2), 200. https://doi.org/10.3390/ph16020200