Dihydromyricetin Modulates Nrf2 and NF-κB Crosstalk to Alleviate Methotrexate-Induced Lung Toxicity
Abstract
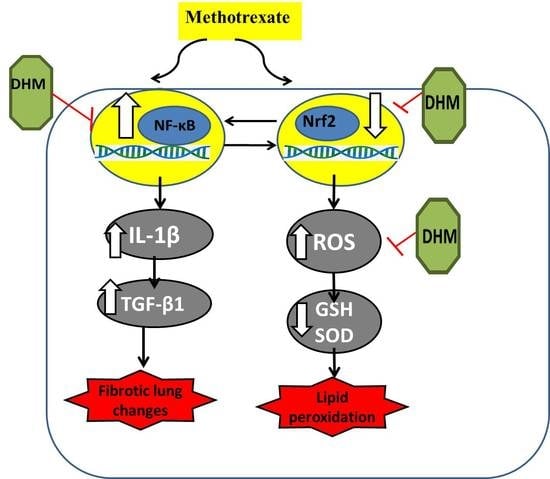
:1. Introduction
2. Results
2.1. Dihydromyricetin Decreased MTX-Induced Weight Loss
2.2. Dihydromyricetin Attenuated MTX-Induced Histopathological Changes in Lung Tissues
2.3. Dihydromyricetin Enhanced the Antioxidant Enzymes while Attenuating MDA Levels in Lung Tissues of MTX-Treated Rats
2.4. DHM Downregulated NF-κB while Upregulating Nrf2/HO-1 Expression in Lung Tissues of MTX-Treated Rats
2.5. DHM Alleviated the MTX-Induced Elevation of IL-1β and TGFβ-1
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals and Experimental Design
4.3. Histological Examination
4.4. Measurement of Oxidative Stress Parameters
4.5. Measurement of Nrf2, HO-1, NF-κB, IL-1β, and TGF-β1 Levels in Lung Tissues Using Enzyme-Linked Immunoassay (ELISA) Technique
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kremer, J.M. Toward a better understanding of methotrexate. Arthritis Rheum. 2004, 50, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, Y.; Guillot, X.; Selambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef] [Green Version]
- Kozminski, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. Low-dose methotrexate: A mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 2005, 57, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Sakthiswary, R.; Suresh, E. Methotrexate in systemic lupus erythematosus: A systematic review of its efficacy. Lupus 2014, 23, 225–235. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Ainsworth, M.A.; Steenholdt, C. Methotrexate for inflammatory bowel disease: Time for reconsideration. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 407–409. [Google Scholar] [CrossRef]
- Sramek, M.; Neradil, J.; Sterba, J.; Veselska, R. Non-DHFR-mediated effects of methotrexate in osteosarcoma cell lines: Epigenetic alterations and enhanced cell differentiation. Cancer Cell Int. 2016, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Matouk, A.I.; Awad, E.M.; El-Tahawy, N.F.G.; El-Sheikh, A.A.K.; Waz, S. Dihydromyricetin alleviates methotrexate-induced hepatotoxicity via suppressing the TLR4/NF-kappaB pathway and NLRP3 inflammasome/caspase 1 axis. Biomed. Pharmacother. 2022, 155, 113752. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Nikiphorou, E.; Larsen, J.; Korsten, P.; Conway, R. Methotrexate-Associated Pneumonitis and Rheumatoid Arthritis-Interstitial Lung Disease: Current Concepts for the Diagnosis and Treatment. Front. Med. 2019, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Al Nokhatha, S.A.; Harrington, R.; Conway, R. Is methotrexate contra-indicated in lung involvement of rheumatoid arthritis? Jt. Bone Spine 2020, 87, 535–537. [Google Scholar] [CrossRef]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Jakubovic, B.D.; Donovan, A.; Webster, P.M.; Shear, N.H. Methotrexate-induced pulmonary toxicity. Can. Respir. J. 2013, 20, 153–155. [Google Scholar] [CrossRef]
- Thaniyan, A.; Ayman, F.F.A.; Mirghani, H.O.; Al-Sayed, B.A.; Merghani, T.H. Histopathological Features of Methotrexate Induced Pulmonary Lesions in Rheumatoid Arthritis Patients: A Systematic Review of Case Reports. Open Access Maced. J. Med. Sci. 2017, 5, 266–270. [Google Scholar] [CrossRef]
- Koyama, S.; Sato, E.; Takamizawa, A.; Tsukadaira, A.; Haniuda, M.; Kurai, M.; Numanami, H.; Nagai, S.; Izumi, T. Methotrexate stimulates lung epithelial cells to release inflammatory cell chemotactic activities. Exp. Lung Res. 2003, 29, 91–111. [Google Scholar] [CrossRef]
- Sato, E.; Camhi, S.L.; Koyama, S.; Robbins, R.A. Methotrexate stimulates lung fibroblasts and epithelial cells to release eosinophil chemotactic activity. J. Rheumatol. 2001, 28, 502–508. [Google Scholar] [PubMed]
- Kurt, A.; Tumkaya, L.; Turut, H.; Cure, M.C.; Cure, E.; Kalkan, Y.; Sehitoglu, I.; Acipayam, A. Protective Effects of Infliximab on Lung Injury Induced by Methotrexate. Arch. De Bronconeumol. 2015, 51, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, R.; Suleyman, B.; Akturan, S.; Cimen, F.K.; Kurt, N.; Suleyman, Z.; Malkoc, I. Effect of lutein on methotrexate-induced oxidative lung damage in rats: A biochemical and histopathological assessment. Korean J. Intern. Med. 2019, 34, 1279–1286. [Google Scholar] [CrossRef]
- Arpag, H.; Gul, M.; Aydemir, Y.; Atilla, N.; Yigitcan, B.; Cakir, T.; Polat, C.; Thornehirli, O.; Sayan, M. Protective Effects of Alpha-Lipoic Acid on Methotrexate-Induced Oxidative Lung Injury in Rats. J. Investig. Surg. 2018, 31, 107–113. [Google Scholar] [CrossRef]
- Shen, Y.; Lindemeyer, A.K.; Gonzalez, C.; Shao, X.M.; Spigelman, I.; Olsen, R.W.; Liang, J. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zeng, M.; Sun, R.; Hu, M. Disposition of flavonoids impacts their efficacy and safety. Curr. Drug Metab. 2014, 15, 841–864. [Google Scholar] [CrossRef]
- Liu, B.; Tan, X.; Liang, J.; Wu, S.; Liu, J.; Zhang, Q.; Zhu, R. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci. Rep. 2014, 4, 7041. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Liu, J.; Chen, H.; Liu, B.; Zhang, Q.; Li, M.; Zhu, R. Dihydromyricetin induces cell cycle arrest and apoptosis in melanoma SK-MEL-28 cells. Oncol. Rep. 2014, 31, 2713–2719. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.W.; Park, N.S.; Noh, M.H.; Shim, J.A.; Ahn, B.N.; Kim, Y.S.; Kim, D.; Lee, H.K.; Hur, D.Y. Combination treatment with erlotinib and ampelopsin overcomes erlotinib resistance in NSCLC cells via the Nox2-ROS-Bim pathway. Lung Cancer 2017, 106, 115–124. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Chan, H.F.; Lu, H.; Lin, Z.; He, C.; Chen, M. Dihydromyricetin Induces Apoptosis and Reverses Drug Resistance in Ovarian Cancer Cells by p53-mediated Downregulation of Survivin. Sci. Rep. 2017, 7, 46060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Luo, P.; Fu, Y.; Wang, J.; Dai, J.; Shao, J.; Yang, X.; Chang, L.; Weng, Q.; Yang, B.; et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.X.; Zhao, Y.F.; Cao, T.; Zhen, X.C. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol. Sin. 2016, 37, 1315–1324. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, X.; Xia, T.; Bi, Y.; Liu, B.; Fu, J.; Zhu, R. Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease. Biomed. Pharmacother. 2021, 142, 111927. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.Q.; Sun, L.L.; Xu, M.T.; Yu, J.; Liu, L.L.; Zhang, J.Y.; Wang, Y.Q.; Wang, H.X.; Bao, X.F.; et al. Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. Int. J. Mol. Sci. 2018, 19, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Tan, K.; Luo, Q.; Bai, X. Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn J. Basic Med. Sci. 2020, 20, 372–380. [Google Scholar] [CrossRef]
- Liu, T.T.; Zeng, Y.; Tang, K.; Chen, X.; Zhang, W.; Xu, X.L. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 2017, 262, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Jiang, B.; Wan, W.; Zhai, W.; Xu, L.; Hu, K.; Xiao, P. Metabolomics reveals the protective of Dihydromyricetin on glucose homeostasis by enhancing insulin sensitivity. Sci. Rep. 2016, 6, 36184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; He, J.; Chen, H.; Yan, H.; Luo, Y.; Huang, Z. Dihydromyricetin Enhances Intestinal Antioxidant Capacity of Growing-Finishing Pigs by Activating ERK/Nrf2/HO-1 Signaling Pathway. Antioxidants 2022, 11, 704. [Google Scholar] [CrossRef]
- Chu, J.; Wang, X.; Bi, H.; Li, L.; Ren, M.; Wang, J. Dihydromyricetin relieves rheumatoid arthritis symptoms and suppresses expression of pro-inflammatory cytokines via the activation of Nrf2 pathway in rheumatoid arthritis model. Int. Immunopharmacol. 2018, 59, 174–180. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.J.; Gu, C.; Zhu, T.; Luo, Y.Y.; Pang, M.X.; Du, W.F.; Ge, W.H. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xiao, Y.; Yang, X.; He, Y.; Jing, T.; Wang, W.; Zhang, J.; Lin, R. Protective effect of dihydromyricetin on LPS-induced acute lung injury. J. Leukoc. Biol. 2018, 103, 1241–1249. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, Q.X.; Zheng, Q.; Liu, T.; Xu, X.E.; Liu, X.H.; Gao, W.; Bai, X.J.; Li, Z.F. Dihydromyricetin Alleviates Sepsis-Induced Acute Lung Injury through Inhibiting NLRP3 Inflammasome-Dependent Pyroptosis in Mice Model. Inflammation 2019, 42, 1301–1310. [Google Scholar] [CrossRef]
- Boukhettala, N.; Leblond, J.; Claeyssens, S.; Faure, M.; Le Pessot, F.; Bole-Feysot, C.; Hassan, A.; Mettraux, C.; Vuichoud, J.; Lavoinne, A.; et al. Methotrexate induces intestinal mucositis and alters gut protein metabolism independently of reduced food intake. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E182–E190. [Google Scholar] [CrossRef]
- Zaki, S.M.; Hussein, G.H.A.; Khalil, H.M.A.; Abd Algaleel, W.A. Febuxostat ameliorates methotrexate-induced lung damage. Folia Morphol. 2021, 80, 392–402. [Google Scholar] [CrossRef]
- Chikura, B.; Sathi, N.; Lane, S.; Dawson, J.K. Variation of immunological response in methotrexate-induced pneumonitis. Rheumatology 2008, 47, 1647–1650. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, C.; Parrot, A.; Wislez, M.; Prigent, H.; Boussaud, V.; Bernaudin, J.F.; Mayaud, C.; Cadranel, J. Spectrum of CD4 to CD8 T-cell ratios in lymphocytic alveolitis associated with methotrexate-induced pneumonitis. Am. J. Respir. Crit. Care Med. 2001, 164, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Kalemci, S.; Topal, Y.; Celik, S.Y.; Yilmaz, N.; Beydilli, H.; Kosar, M.I.; Dirican, N.; Altuntas, I. Silibinin attenuates methotrexate-induced pulmonary injury by targeting oxidative stress. Exp. Ther. Med. 2015, 10, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalemci, S.; Dirican, N.; Cetin, E.S.; Sozen, H.; Uner, A.G.; Yaylali, A.; Aksun, S.; Karacam, V.; Ulger, E.; Sutcu, R.; et al. The efficacy of minocycline against methotrexate-induced pulmonary fibrosis in mice. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3334–3340. [Google Scholar]
- Jahovic, N.; Cevik, H.; Sehirli, A.O.; Yegen, B.C.; Sener, G. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J. Pineal Res. 2003, 34, 282–287. [Google Scholar] [CrossRef]
- Miyazono, Y.; Gao, F.; Horie, T. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand. J. Gastroenterol. 2004, 39, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Sepand, M.R.; Seyednejad, S.A.; Omidi, A.; Akbariani, M.; Gholami, M.; Sabzevari, O. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IkBalpha/NFkB in rats. Daru 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, T.T.; Zhao, H.Y.; Wang, H. Melatonin protects methotrexate-induced testicular injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7517–7525. [Google Scholar] [CrossRef] [PubMed]
- Aladaileh, S.H.; Hussein, O.E.; Abukhalil, M.H.; Saghir, S.A.M.; Bin-Jumah, M.; Alfwuaires, M.A.; Germoush, M.O.; Almaiman, A.A.; Mahmoud, A.M. Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats. Antioxidants 2019, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; Abd El-Twab, S.M.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARgamma and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. Int. 2020, 27, 20725–20735. [Google Scholar] [CrossRef]
- Younis, N.S.; Elsewedy, H.S.; Shehata, T.M.; Mohamed, M.E. Geraniol Averts Methotrexate-Induced Acute Kidney Injury via Keap1/Nrf2/HO-1 and MAPK/NF-kappaB Pathways. Curr. Issues Mol. Biol. 2021, 43, 1741–1755. [Google Scholar] [CrossRef]
- Kawami, M.; Honda, M.; Hara, T.; Yumoto, R.; Takano, M. Role of Nrf2 in Methotrexate-Induced Epithelial-Mesenchymal Transition in Alveolar A549 Cells. Biol. Pharm. Bull. 2022, 45, 1069–1076. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yi, L.; Zhou, X.; Mi, M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 2018, 44, 123–136. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Wang, H.; Zhang, J.; Xie, L.; Huo, Y.; Ma, W.; Li, H.; Chen, X.; Shi, P. The role of miR-199b-3p in regulating Nrf2 pathway by dihydromyricetin to alleviate septic acute kidney injury. Free. Radic. Res. 2021, 55, 842–852. [Google Scholar] [CrossRef]
- Awad, E.M.; Ahmed, A.F.; El-Daly, M.; Amin, A.H.; El-Tahawy, N.F.G.; Wagdy, A.; Hollenberg, M.D.; Taye, A. Dihydromyricetin protects against high glucose-induced endothelial dysfunction: Role of HIF-1alpha/ROR2/NF-kappaB. Biomed. Pharmacother. 2022, 153, 113308. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Liu, Z.; Yang, K.; Chen, Z.; Cheng, Q.; Wu, L. The Versatile Effects of Dihydromyricetin in Health. Evid.-Based Complement. Altern. Med. 2017, 2017, 1053617. [Google Scholar] [CrossRef] [Green Version]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxidative Med. Cell. Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.; Caumont-Sarcos, A.; Ieva, R. Mitochondrial presequence import: Multiple regulatory knobs fine-tune mitochondrial biogenesis and homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, W.; Wang, Y.; Zhao, Q.; Yu, X.; Jiang, J.; Bian, W.; Xu, C.; Sun, Y.; Zhang, C. The mechanism of rh-endostatin-induced cardiotoxicity and its protection by dihydromyricetin[in vivo/in vitro, C57BL/6 mice, AC16 and hiPSC-CMs]. Toxicol. Lett. 2023, 377, 29–37. [Google Scholar] [CrossRef]
- Kim, Y.J.; Song, M.; Ryu, J.C. Inflammation in methotrexate-induced pulmonary toxicity occurs via the p38 MAPK pathway. Toxicology 2009, 256, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; El-Sheikh, A.A.K.; Abdel-Hafez, S.M.N.; Kandeel, M.; Abdel-Gaber, S.A. Paeonol Protects Against Methotrexate-Induced Nephrotoxicity via Upregulation of P-gp Expression and Inhibition of TLR4/NF-kappaB Pathway. Front. Pharmacol. 2022, 13, 774387. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Scholz, C.C.; Cavadas, M.A.; Tambuwala, M.M.; Hams, E.; Rodriguez, J.; von Kriegsheim, A.; Cotter, P.; Bruning, U.; Fallon, P.G.; Cheong, A.; et al. Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18490–18495. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lin, R.; Zhao, B.; Guan, R.; Li, T.; Jin, R. Correlation between oxidative stress and the NF-kappaB signaling pathway in the pulmonary tissues of obese asthmatic mice. Mol. Med. Rep. 2016, 13, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Ali, Y.A.; Ahmed, A.A.E.; Abd El-Raouf, O.M.; Elkhoely, A.; Gad, A.M. Polydatin combats methotrexate-induced pulmonary fibrosis in rats: Involvement of biochemical and histopathological assessment. J. Biochem. Mol. Toxicol. 2022, 36, e23019. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Wang, H.; Yan, W.; Xu, L.; Wang, X.; Zhao, X.; Yang, X.; Chen, G.; Ji, Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediat. Inflamm. 2008, 2008, 725174. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Sehsah, R.; Wu, W.; Ichihara, S.; Hashimoto, N.; Hasegawa, Y.; Zong, C.; Itoh, K.; Yamamoto, M.; Elsayed, A.A.; El-Bestar, S.; et al. Role of Nrf2 in inflammatory response in lung of mice exposed to zinc oxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Ma, Q. Suppression of basal and carbon nanotube-induced oxidative stress, inflammation and fibrosis in mouse lungs by Nrf2. Nanotoxicology 2016, 10, 699–709. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Ding, C.; Gu, Y.; Liu, W. Dihydromyricetin Reverses Thioacetamide-Induced Liver Fibrosis Through Inhibiting NF-kappaB-Mediated Inflammation and TGF-beta1-Regulated of PI3K/Akt Signaling Pathway. Front. Pharmacol. 2021, 12, 783886. [Google Scholar] [CrossRef]
- Tang, N.; Ma, J.; Wang, K.S.; Mi, C.; Lv, Y.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Dihydromyricetin suppresses TNF-alpha-induced NF-kappaB activation and target gene expression. Mol. Cell Biochem. 2016, 422, 11–20. [Google Scholar] [CrossRef]
- Wu, J.Z.; Ardah, M.; Haikal, C.; Svanbergsson, A.; Diepenbroek, M.; Vaikath, N.N.; Li, W.; Wang, Z.Y.; Outeiro, T.F.; El-Agnaf, O.M.; et al. Dihydromyricetin and Salvianolic acid B inhibit alpha-synuclein aggregation and enhance chaperone-mediated autophagy. Transl. Neurodegener. 2019, 8, 18. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Yang, Y.; Lin, M.; Liu, C.; Wang, W.; Zhou, X.; Ai, Q.; et al. Mechanism of Dihydromyricetin on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 794563. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Pan, J.Q.; Luo, L.; Ning, X.J.; Ye, Z.P.; Yu, Z.; Li, W.S. NF-kappaB induces miR-148a to sustain TGF-beta/Smad signaling activation in glioblastoma. Mol. Cancer 2015, 14, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rameshwar, P.; Narayanan, R.; Qian, J.; Denny, T.N.; Colon, C.; Gascon, P. NF-kappa B as a central mediator in the induction of TGF-beta in monocytes from patients with idiopathic myelofibrosis: An inflammatory response beyond the realm of homeostasis. J. Immunol. 2000, 165, 2271–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawami, M.; Harabayashi, R.; Miyamoto, M.; Harada, R.; Yumoto, R.; Takano, M. Methotrexate-Induced Epithelial-Mesenchymal Transition in the Alveolar Epithelial Cell Line A549. Lung 2016, 194, 923–930. [Google Scholar] [CrossRef]
- Kawami, M.; Harada, R.; Ojima, T.; Yamagami, Y.; Yumoto, R.; Takano, M. Association of cell cycle arrest with anticancer drug-induced epithelial-mesenchymal transition in alveolar epithelial cells. Toxicology 2019, 424, 152231. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kawami, M.; Konaka, T.; Takenaka, S.; Yumoto, R.; Takano, M. Anticancer Drug-Induced Epithelial-Mesenchymal Transition via p53/miR-34a axis in A549/ABCA3 Cells. J. Pharm. Pharm. Sci. 2019, 22, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Mo, X.; Cui, W.; Zhang, Z.; Li, D.; Li, L.; Xu, L.; Yao, H.; Gao, J. Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis. Sci. Rep. 2016, 6, 38646. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Luo, G.; Dodson, M.; Schmidlin, C.J.; Wei, Y.; Kerimoglu, B.; Ooi, A.; Chapman, E.; Garcia, J.G.; Zhang, D.D. The NRF2-LOC344887 signaling axis suppresses pulmonary fibrosis. Redox Biol. 2021, 38, 101766. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J.; Deng, H.; Zheng, L.; Yang, H.; Lv, X. The Role of Nrf2 in Pulmonary Fibrosis: Molecular Mechanisms and Treatment Approaches. Antioxidants 2022, 11, 1685. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, L.; Zhou, M.; Hou, P.; Yi, L.; Mi, M. Dihydromyricetin ameliorates liver fibrosis via inhibition of hepatic stellate cells by inducing autophagy and natural killer cell-mediated killing effect. Nutr. Metab. 2021, 18, 64. [Google Scholar] [CrossRef]
- Wang, J.T.; Jiao, P.; Zhou, Y.; Liu, Q. Protective Effect of Dihydromyricetin Against Lipopolysaccharide-Induced Acute Kidney Injury in a Rat Model. Med. Sci. Monit. 2016, 22, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Z.; Li, H.; Zhu, N.; Gu, J.; Wang, W.; Liu, C.; Wang, W.; Qin, L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers 2022, 14, 3487. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ibrahim, S.A.; Eltahawy, N.F.; Abdalla, A.M.; Khalaf, H.M. Protective effects of selenium in tacrolimus-induced lung toxicity: Potential role of heme oxygenase 1. Can. J. Physiol. Pharmacol. 2021, 99, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, M. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest Med. 1982, 3, 35–56. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]




| Control (g) | MTX (g) | MTX + DHM (g) | DHM (g) | |
|---|---|---|---|---|
| At 0 days | 242.5 ± 11.09 | 230.0 ± 10.8 | 240.0 ± 9.13 | 226.3 ± 11.43 |
| At 14 days | 290.0 ± 9.13 | 196.5 ± 5.96 * | 246.5 ± 6.24 # | 275.8 ± 6.3 |
| Parameter | Groups | |||
|---|---|---|---|---|
| Control | MTX | MTX + DHM | DHM | |
| Consolidation | 0 | 1.8 * | 0 # | 0 |
| Interalveolar septa thickening | 0 | 3.0 * | 1.4 # | 0 |
| Vascular congestion | 0 | 3.3 * | 1.0 # | 0 |
| Interstitial hemorrhage | 0 | 2.0 * | 0.5 # | 0 |
| Inflammatory cell infiltration | 0 | 3.0 * | 1 # | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matouk, A.I.; Awad, E.M.; El-Tahawy, N.F.G.; El-Sheikh, A.A.K.; Anter, A. Dihydromyricetin Modulates Nrf2 and NF-κB Crosstalk to Alleviate Methotrexate-Induced Lung Toxicity. Pharmaceuticals 2023, 16, 481. https://doi.org/10.3390/ph16040481
Matouk AI, Awad EM, El-Tahawy NFG, El-Sheikh AAK, Anter A. Dihydromyricetin Modulates Nrf2 and NF-κB Crosstalk to Alleviate Methotrexate-Induced Lung Toxicity. Pharmaceuticals. 2023; 16(4):481. https://doi.org/10.3390/ph16040481
Chicago/Turabian StyleMatouk, Asmaa I., Eman M. Awad, Nashwa F. G. El-Tahawy, Azza A. K. El-Sheikh, and Aliaa Anter. 2023. "Dihydromyricetin Modulates Nrf2 and NF-κB Crosstalk to Alleviate Methotrexate-Induced Lung Toxicity" Pharmaceuticals 16, no. 4: 481. https://doi.org/10.3390/ph16040481
APA StyleMatouk, A. I., Awad, E. M., El-Tahawy, N. F. G., El-Sheikh, A. A. K., & Anter, A. (2023). Dihydromyricetin Modulates Nrf2 and NF-κB Crosstalk to Alleviate Methotrexate-Induced Lung Toxicity. Pharmaceuticals, 16(4), 481. https://doi.org/10.3390/ph16040481