Fabrication and Characterization of Celecoxib-Loaded Chitosan/Guar Gum-Based Hydrogel Beads
Abstract
:1. Introduction
2. Results
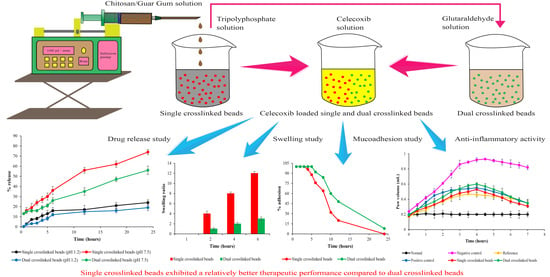
2.1. Preparation of Tripolyphosphate and Glutaraldehyde Crosslinked Chitosan/Guar Gum-Based Hydrogel Beads
2.2. Optimization of Single and Dual Crosslinked Hydrogel Beads
2.2.1. Particle Size
2.2.2. Swelling Studies
2.2.3. Optimization
2.3. Entrapment and Loading Efficiency
2.4. Scanning Electron Microscopy
2.5. Fourier Transform Infrared Spectroscopy
2.6. X-ray Diffraction
2.7. Thermal Analysis
2.8. In Vitro Drug Release Studies
2.9. Ex Vivo Studies
2.9.1. Mucoadhesion studies
2.9.2. Permeability Studies
2.9.3. Swelling Studies
2.10. In Vivo Anti-Inflammatory Activity
2.10.1. Determination of Inflammatory Markers
2.10.2. Histopathological Examination of Paw Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Sodium Tripolyphosphate and Glutaraldehyde Crosslinked Chitosan/Guar Gum-Based Hydrogel Beads
4.2.1. Preparation of Single Crosslinked Hydrogel Beads
4.2.2. Preparation of Dual Crosslinked Hydrogel Beads
4.3. Preparation of Celecoxi-Loaded Hydrogel Beads
4.4. Optimization of Single and Dual Crosslinked Hydrogel Beads
4.4.1. Particle Size
4.4.2. Swelling Studies
4.4.3. Experimental Design
4.5. Entrapment and Loading Efficiency
4.6. Scanning Electron Microscopy
4.7. Fourier Transform Infrared Spectroscopy
4.8. X-ray Diffraction
4.9. Thermal Analysis
4.10. In Vitro Drug Release Studies
4.11. Ex Vivo Studies
4.11.1. Mucoadhesion Studies
4.11.2. Permeability Studies
4.11.3. Swelling Studies
4.12. In Vivo Anti-Inflammatory Activity
4.12.1. Induction of Carrageenan-Induced Rat Paw Oedema
4.12.2. Study Design
4.12.3. Determination of Inflammatory Markers
4.12.4. Histopathological Examination of Paw Tissues
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full form/explanation |
| CS | Chitosan |
| GG | Guar gum |
| SC | Single crosslinked beads |
| DC | Dual/double crosslinked beads |
| EE% | Entrapment efficiency |
| LE% | Loading efficiency |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
| CX | Celecoxib |
| NSAID | Non-steroidal anti-inflammatory drug |
| GIT | Gastrointestinal tract |
| STPP/TPP | Sodium tripolyphosphate |
| GLA | Glutaraldehyde |
| PO4− | Phosphate group |
| NH3+ | Amino group |
| -OH | Hydroxyl group |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| Papp | Apparent permeability coefficient |
| CG | Carrageenan |
| S(EQ) | Equilibrium swelling ratio |
References
- Segale, L.; Mannina, P.; Giovannelli, L.; Pattarino, F. Calcium alginate multi-unit oral dosage form for delayed release of celecoxib. J. Drug Deliv. Sci. Technol. 2015, 26, 35–43. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, D.H.; Nguyen, D.T.; Lee, H.S.; Kang, N.W.; Baek, M.J.; An, J.; Yoo, S.Y.; Mun, Y.H.; Lee, W.; et al. Preparation and Evaluation of Eudragit L100-PEG Proliponiosomes for Enhanced Oral Delivery of Celecoxib. Pharmaceutics 2020, 12, 718. [Google Scholar] [CrossRef]
- Jain, S.K.; Jain, A.; Gupta, Y.; Ahirwar, M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech 2007, 8, E34–E41. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.S.; Mujeeb, M.; Zafar, S.; Khan, W.Q.; Patel, M.; Yousef, B.; Chang, M.W.; Sayed, E.; Ahmad, Z. EHDA engineering of Piroxicam-PVP components for pharmaceutical dosages. J. Drug Deliv. Sci. Technol. 2022, 78, 103927. [Google Scholar] [CrossRef]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel beads for designing future foods: Structures, mechanisms, applications, and challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Mun, A.; Simaan Yameen, H.; Edelbaum, G.; Seliktar, D. Alginate hydrogel beads embedded with drug-bearing polycaprolactone microspheres for sustained release of paclobutrazol. Sci. Rep. 2021, 11, 10877. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Jing, J.; Yang, F. Cellulose/guar gum hydrogel microspheres as a magnetic anticancer drug carrier. BioResources 2019, 14, 3615–3629. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Siepmann, F.; Siepmann, J.; Pongjanyakul, T. Chitosan-clay nanocomposite microparticles for controlled drug delivery: Effects of the MAS content and TPP crosslinking. J. Drug Deliv. Sci. Technol. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Galan, J.; Trilleras, J.; Zapata, P.A.; Arana, V.A.; Grande-Tovar, C.D. Optimization of Chitosan Glutaraldehyde-Crosslinked Beads for Reactive Blue 4 Anionic Dye Removal Using a Surface Response Methodology. Life 2021, 11, 85. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.J.; Khalid, M.; Jamil, T.; Aftab, S.; Mangat, S.A.; Shakoori, A.R.; Iqbal, S. Formulation of novel chitosan guargum based hydrogels for sustained drug release of paracetamol. Int. J. Biol. Macromol. 2018, 108, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Manjanna, K.M.; Rajesh, K.S.; Shivakumar, B. Formulation and Optimization of Natural Polysaccharide Hydrogel Microbeads of Aceclofenac Sodium for Oral Controlled Drug Delivery. Am. J. Med. Sci. Med. 2023, 1, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Budianto, E.; Muthoharoh, S.P.; Nizardo, N.M. Effect of Crosslinking Agents, pH and Temperature on Swelling Behavior of Cross-linked Chitosan Hydrogel. Asian J. Appl. Sci. 2015, 03, 2321–2893. [Google Scholar]
- Sedyakina, N.; Kuskov, A.; Velonia, K.; Feldman, N.; Lutsenko, S.; Avramenko, G. Modulation of Entrapment Efficiency and in Vitro Release Properties of BSA-Loaded Chitosan Microparticles Cross-Linked with Citric Acid as a Potential Protein–Drug Delivery System. Materials 2020, 13, 1989. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerma, A.G.; Morales-Vargas, A.T.; Rodríguez-Núñez, J.R. Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- Daud, H.; Ghani, A.; Iqbal, D.N.; Ahmad, N.; Nazir, S.; Muhammad, M.J.; Hussain, E.A.; Nazir, A.; Iqbal, M. Preparation and characterization of guar gum based biopolymeric hydrogels for controlled release of antihypertensive drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Dinari, M.; Bina, F.; Khayamian, T. Poly (vinyl alcohol)-based electrospun nanofibers for the sustained release of celecoxib: Characterization and evaluation of drug release mechanism. Polym. Compos. 2018, 39, E221–E227. [Google Scholar] [CrossRef]
- Mao, C.; Imtiaz, S.A.; Zhang, Y. Competitive adsorption of Ag (I) and Cu (II) by tripolyphosphate crosslinked chitosan beads. J. Appl. Polym. Sci. 2015, 132, 42717. [Google Scholar] [CrossRef]
- Liu, B.; Chen, W.; Peng, X.; Cao, Q.; Wang, Q.; Wang, D.; Meng, X.; Yu, G. Biosorption of lead from aqueous solutions by ion-imprinted tetraethylenepentamine modified chitosan beads. Int. J. Biol. Macromol. 2016, 86, 562–569. [Google Scholar] [CrossRef]
- Arshad, M.S.; Hassan, S.; Hussain, A.; Abbas, N.; Kucuk, I.; Nazari, K.; Ali, R.; Ramzan, S.; Alqahtani, A.; Andriotis, E.G.; et al. Improved transdermal delivery of cetirizine hydrochloride using polymeric microneedles. DARU J. Pharm. Sci. 2019, 27, 673–681. [Google Scholar] [CrossRef]
- Eswaramma, S.; Rao, K.S.V.K. Synthesis of dual responsive carbohydrate polymer based IPN microbeads for controlled release of anti-HIV drug. Carbohydr. Polym. 2017, 156, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Karzar Jeddi, M.; Mahkam, M. Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef]
- Arslan, A.; Yet, B.; Nemutlu, E.; Akdağ Çaylı, Y.; Eroğlu, H.; Öner, L. Celecoxib nanoformulations with enhanced solubility, dissolution rate, and oral bioavailability: Experimental approaches over in vitro/in vivo Evaluation. Pharmaceutics 2023, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Mujtaba, A.; Kohli, K. Lipid drug conjugate nanoparticle as a potential nanocarrier for the oral delivery of pemetrexed diacid: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Biol. Macromol. 2017, 103, 139–151. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. BioImpacts BI 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.; Ranjha, N.M.; Shoukat, H.; Madni, A.; Ahmad, F.; Raza, M.R.; Jameel, Q.A.; Majeed, A.; Ramzan, N. Fabrication, Evaluation, In Vivo Pharmacokinetic and Toxicological Analysis of pH-Sensitive Eudragit S-100-Coated Hydrogel Beads: A Promising Strategy for Colon Targeting. AAPS PharmSciTech 2021, 22, 209. [Google Scholar] [CrossRef]
- Begum, M.Y.; Alqahtani, A.; Ghazwani, M.; Ramakrishna, M.M.; Hani, U.; Atiya, A.; Rahamathulla, M. Preparation of Carbopol 934 Based Ketorolac Tromethamine Buccal Mucoadhesive Film: In Vitro, Ex Vivo, and in Vivo Assessments. Int. J. Polym. Sci. 2021, 2021, 4786488. [Google Scholar] [CrossRef]
- Malkani, A.; Date, A.A.; Hegde, D. Celecoxib nanosuspension: Single-step fabrication using a modified nanoprecipitation method and in vivo evaluation. Drug Deliv. Transl. Res. 2014, 4, 365–376. [Google Scholar] [CrossRef]
- Vazquez, E.; Navarro, M.; Salazar, Y.; Crespo, G.; Bruges, G.; Osorio, C.; Tortorici, V.; Vanegas, H.; López, M. Systemic changes following carrageenan-induced paw inflammation in rats. Inflamm. Res. 2015, 64, 333–342. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, D.H.; Ahn, J.B.; Sim, S.; Heo, K.S.; Myung, C.S.; Park, J.S. Therapeutic effects of celecoxib polymeric systems in rat models of inflammation and adjuvant-induced rheumatoid arthritis. Mater. Sci. Eng. C 2020, 114, 111042. [Google Scholar] [CrossRef]
- Arshad, M.S.; Zafar, S.; Yousef, B.; Alyassin, Y.; Ali, R.; AlAsiri, A.; Chang, M.-W.; Ahmad, Z.; Elkordy, A.A.; Faheem, A.; et al. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv. Drug Deliv. Rev. 2021, 178, 13840. [Google Scholar]
- Chen, S.; Zhu, H.; Luo, Y. Chitosan-based oral colon-specific delivery systems for polyphenols: Recent advances and emerging trends. J. Mater. Chem. B 2022, 10, 7328–7348. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Wadhwa, S.; Srivastava, A.K. Cross-Linked Guar Gum Hydrogel Discs for Colon-Specific Delivery of Ibuprofen: Formulation and In Vitro Evaluation. Drug Deliv. 2008, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The role of oral controlled release matrix tablets in drug delivery systems. Bioimpacts 2012, 2, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewinska, D. Effect of electrospinning process variables on the size of polymer fibers and bead-on-string structures established with a 23 factorial design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.; Ali Chohan, T.; Mudassir, J.; Mehta, P.; Yousef, B.; Zaman, A.; Ali, A.; Qutachi, O.; Chang, M.W.; Fatouros, D.; et al. Evaluation of sustained-release in-situ injectable gels, containing naproxen sodium, using in vitro, in silico and in vivo analysis. Int. J. Pharm. 2022, 616, 121512. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef] [PubMed]














| Time (h) | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 10 | 12 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|
| % Adhesion SC5 | 100 ± 00 | 100 ± 00 | 100 ± 0.57 | 96 ± 0.55 | 88 ± 00 | 76 ± 0.57 | 64 ± 0.57 | 32 ± 00 | 20 ± 0.52 | 0 ± 0.5 |
| % Adhesion DC5 | 100 ± 00 | 100 ± 00 | 100 ± 00 | 100 ± 00 | 100 ± 0.57 | 92 ± 0.57 | 84 ± 0.57 | 60 ± 00 | 48 ± 0.57 | 8 ± 00 |
| Variable Parameters | ||||
|---|---|---|---|---|
| Formulation Code | CS (% w/v) | GG (% w/v) | ||
| L | SC1 | 1.5 (−1) | 10 (−1) | |
| SC2 | 1.5 (−1) | 30 (0) | ||
| SC3 | 1.5 (−1) | 50 (1) | ||
| M | SC4 | 2 (0) | 10 (−1) | |
| SC5 | 2 (0) | 30 (0) | ||
| SC6 | 2 (0) | 50 (1) | ||
| H | SC7 | 2.5 (1) | 10 (−1) | |
| SC8 | 2.5 (1) | 30 (0) | ||
| SC9 | 2.5 (1) | 50 (1) | ||
| L | DC1 | 1.5 (−1) | 10 (−1) | |
| DC2 | 1.5 (−1) | 30 (0) | ||
| DC3 | 1.5 (−1) | 50 (1) | ||
| M | DC4 | 2 (0) | 10 (−1) | |
| DC5 | 2 (0) | 30 (0) | ||
| DC6 | 2 (0) | 50 (1) | ||
| H | DC7 | 2.5 (1) | 10 (−1) | |
| DC8 | 2.5 (1) | 30 (0) | ||
| DC9 | 2.5 (1) | 50 (1) | ||
| Constant Parameters (SC & DC) L (Low), M (Medium) & H (High) | ||||
| Total volume (polymer solution) (mL) | TPP 1% volume (mL) | Stirring speed (rpm) | GLA 2% volume (mL) | |
| SC | DC | |||
| 10 | 100 | 2000 | 0 | 10 |
| where, for CS content (% w/v), −1 = 1.5, 0 = 2 and 1 = 2.5, and for GG content (% w/v), −1 = 10, 0 = 30 and 1 = 50 | ||||
| Group | Codes | Description | CG (0.1 mL, 1%) Administration | Design of Treatment | Treatment Dose |
|---|---|---|---|---|---|
| 1 | NR | Normal (healthy) | No | No Treatment | 0 |
| 2 | NC | Negative Control | Yes | No Treatment | 0 |
| 3 | RF | Reference | Yes | Pure Celecoxib | 15 mg/kg |
| 4 | PC | Positive Control | Yes | Celbex Capsule | 15 mg/kg |
| 5 | SC | Single Crosslinked Hydrogel Beads | Yes | CX-loaded SC Hydrogel Beads | 15 mg/kg |
| 6 | DC | Dual Crosslinked Hydrogel Beads | Yes | CX-loaded DC Hydrogel Beads | 15 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, R.; Mudassir, J.; Khan, M.A.; Zafar, S.; Rana, S.J.; Abbas, N.; Hussain, A.; Arshad, M.S.; Muhammad, S. Fabrication and Characterization of Celecoxib-Loaded Chitosan/Guar Gum-Based Hydrogel Beads. Pharmaceuticals 2023, 16, 554. https://doi.org/10.3390/ph16040554
Batool R, Mudassir J, Khan MA, Zafar S, Rana SJ, Abbas N, Hussain A, Arshad MS, Muhammad S. Fabrication and Characterization of Celecoxib-Loaded Chitosan/Guar Gum-Based Hydrogel Beads. Pharmaceuticals. 2023; 16(4):554. https://doi.org/10.3390/ph16040554
Chicago/Turabian StyleBatool, Rukhsana, Jahanzeb Mudassir, Mahtab Ahmad Khan, Saman Zafar, Sadia Jafar Rana, Nasir Abbas, Amjad Hussain, Muhammad Sohail Arshad, and Sajjad Muhammad. 2023. "Fabrication and Characterization of Celecoxib-Loaded Chitosan/Guar Gum-Based Hydrogel Beads" Pharmaceuticals 16, no. 4: 554. https://doi.org/10.3390/ph16040554
APA StyleBatool, R., Mudassir, J., Khan, M. A., Zafar, S., Rana, S. J., Abbas, N., Hussain, A., Arshad, M. S., & Muhammad, S. (2023). Fabrication and Characterization of Celecoxib-Loaded Chitosan/Guar Gum-Based Hydrogel Beads. Pharmaceuticals, 16(4), 554. https://doi.org/10.3390/ph16040554