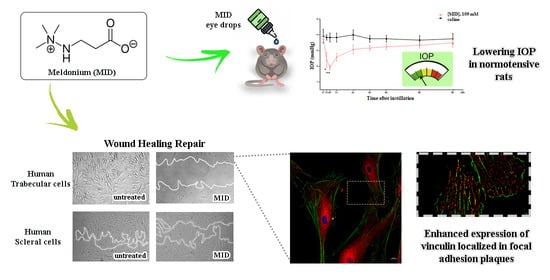
Meldonium Inhibits Cell Motility and Wound-Healing in Trabecular Meshwork Cells and Scleral Fibroblasts: Possible Applications in Glaucoma
Abstract
:1. Introduction
2. Results
2.1. Effect of MID Eye Drops on IOP in Normotensive Rats: In Vivo Study
2.2. Effect of MID on Cell Viability and Apoptosis Induction of HTMC
2.3. Effect of MID on Cytoskeleton Organization
2.4. MID Prevents Wound Repair in Human Trabecular and Scleral Cell Lines
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Treatment with Meldonium Eye Drops
4.3. Cell Culture
4.4. Cellular Metabolic Activity—MTT Assay
4.5. Cell Migration Analyses
4.6. Measurement of Apoptosis by Annexin V Staining
4.7. Immunofluorescence
4.8. Immunoblot
4.9. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarado, J.A.; Betanzos, A.; Franse-Carman, L.; Chen, J.; González-Mariscal, L. Endothelia of Schlemm’s canal and trabecular meshwork: Distinct molecular, functional, and anatomic features. Am. J. Physiol.—Cell Physiol. 2004, 286, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, H. Causal inference in primary open angle glaucoma: Specific discussion on intraocular pressure. Ophthalmic Epidemiol. 2006, 13, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Abu-hassan, D.W.; Acott, T.S.; Kelley, M.J. The Trabecular Meshwork: A Basic Review of Form and Function. J. Ocul. Biol. 2014, 2, 9. [Google Scholar] [CrossRef]
- Tamm, E.R.; Braunger, B.M.; Fuchshofer, R. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 134, ISBN 9780128010594. [Google Scholar]
- Boland, M.V.; Ervin, A.-M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative Effectiveness of Pharmacologic Treatments for Open-Angle Glaucoma: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef]
- Wolfram, C.; Stahlberg, E.; Pfeiffer, N. Patient-reported nonadherence with glaucoma therapy. J. Ocul. Pharmacol. Ther. 2019, 35, 223–228. [Google Scholar] [CrossRef]
- Canut, M.I.; Villa, O.; Kudsieh, B.; Mattlin, H.; Banchs, I.; González, J.R.; Armengol, L.; Casaroli-Marano, R.P. Publisher Correction: MLIP genotype as a predictor of pharmacological response in primary open-angle glaucoma and ocular hypertension. (Scientific Reports, (2021), 11, 1, (1583), 10.1038/s41598-020-80954-2). Sci. Rep. 2021, 11, 8237. [Google Scholar] [CrossRef]
- Chang, P.Y.; Wang, J.Y.; Wang, J.K.; Huang, T.L.; Hsu, Y.R. Comparison of treatment outcomes of selective laser trabeculoplasty for primary open-angle glaucoma and pseudophakic primary angle-closure glaucoma receiving maximal medical therapy. J. Clin. Med. 2021, 10, 2853. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef]
- Wolters, J.E.J.; Van Mechelen, R.J.S.; Al Majidi, R.; Pinchuk, L.; Webers, C.A.B.; Beckers, H.J.M.; Gorgels, T.G.M.F. History, presence, and future of mitomycin C in glaucoma filtration surgery. Curr. Opin. Ophthalmol. 2021, 32, 148–159. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Ferreira, J.d.L.M. Overview of cicatricial modulators in glaucoma fistulizing surgery. Int. Ophthalmol. 2020, 40, 2789–2796. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Zhao, J.; Li, D.; Yan, X.; Liu, J.; Liu, X.; Zhao, H.; Xia, J.; Zhang, X.; et al. Efficacy and safety of mildronate for acute ischemic stroke: A randomized, double-blind, active-controlled phase II multicenter trial. Clin. Drug Investig. 2013, 33, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sjakste, N.; Kalvinsh, I. Mildronate: An antiischemic drug with multiple indications. Pharmacologyonline 2006, 1, 1–18. [Google Scholar]
- Simkhovich, B.Z.; Shutenko, Z.V.; Meirena, D.V.; Khagi, K.B.; Mežapuķe, R.J.; Molodchina, T.N.; Kalvlņš, I.J.; Lukevics, E. 3-(2,2,2-Trimethylhydrazinium)propionate(thp)-a novel γ-butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochem. Pharmacol. 1988, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.W.; Gil, D.W. The inflow and outflow of anti-glaucoma drugs. Trends Pharmacol. Sci. 2004, 25, 238–241. [Google Scholar] [CrossRef]
- Saha, B.C.; Kumari, R.; Kushumesh, R.; Ambasta, A.; Sinha, B.P. Status of Rho kinase inhibitors in glaucoma therapeutics—An overview. Int. Ophthalmol. 2022, 42, 281–294. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in glaucoma: Old and new promising treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- Mierke, C.T. The role of vinculin in the regulation of the mechanical properties of cells. Cell Biochem. Biophys. 2009, 53, 115–126. [Google Scholar] [CrossRef]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys. J. 2008, 94, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, W.H.; Schindl, M.; Cardozo, T.J.; Ezzell, R.M. Motility of vinculin-deficient F9 embryonic carcinoma cells analyzed by video, laser confocal, and reflection interference contrast microscopy. Exp. Cell Res. 1995, 221, 311–319. [Google Scholar] [CrossRef]
- Ezzell, R.M.; Goldmann, W.H.; Wang, N.; Parasharama, N.; Ingber, D.E. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res. 1997, 231, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Curtis Shoyer, T.; Gates, E.M.; Cabe, J.I.; Conway, D.E.; Hoffman, B.D.; Hoffman, B. Coupling During Collective Cell Migration is Controlled by a Vinculin Mechanochemical Switch. bioRxiv 2023, 2023.01.13.523997. [Google Scholar]
- Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Parsadaniantz, S.M.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Khaw, P.T.; Doyle, J.W.; Sherwood, M.B.; Grierson, I.; Schultz, G.; Mcgorray, S. Prolonged Localized Tissue Effects From 5-Minute Exposures to Fluorouracil and Mitomycin C. Arch. Ophthalmol. 1993, 111, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Pitha, Y. Preventing Postoperative Fibrosis. Glaucoma Today 2019, 43–45. [Google Scholar]
- Kirwan, J.F.; Rennie, C.; Evans, J.R. Beta radiation for glaucoma surgery. Sao Paulo Med. J. 2012, 130, 209. [Google Scholar] [CrossRef] [Green Version]
- Gaskin, J.C.F.; Nguyen, D.Q.; Ang, G.S.; O’Connor, J.; Crowston, J.G. Wound healing modulation in glaucoma filtration surgery—Conventional practices and new perspectives: Antivascular endothelial growth factor and novel agents (part II). J. Curr. Glaucoma Pract. 2014, 8, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Swann, F.B.; Singh, S.; Blake, D.; John, V.; Le, C.; Fullerton, M.; Margo, C.; Zhang, Z.; Muddasani, N.; Wall, J.; et al. Effect of 2 Novel Sustained-release Drug Release Systems on Bleb Fibrosis: An in Vivo Trabeculectomy Study in a Rabbit Model. J. Glaucoma 2019, 28, 512–518. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Vilskersts, R.; Makarova, E.; Kuka, J.; Liepinsh, E. Pharmacological effects of meldonium: Biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol. Res. 2016, 113, 771–780. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Misuse of the metabolic modulator meldonium in sports. J. Sport Heal. Sci. 2017, 6, 49–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schobersberger, W.; Dünnwald, T.; Gmeiner, G.; Blank, C. Story behind meldonium-from pharmacology to performance enhancement: A narrative review. Br. J. Sports Med. 2017, 51, 22–25. [Google Scholar] [CrossRef]
- Berlato, D.G.; Bairros, A.V.d. Meldonium: Pharmacological, toxicological, and analytical aspects. Toxicol. Res. Appl. 2020, 4, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rabin, O.; Uiba, V.; Miroshnikova, Y.; Zabelin, M.; Samoylov, A.; Karkischenko, V.; Semyonov, S.; Astrelina, T.; Razinkin, S. Meldonium long-term excretion period and pharmacokinetics in blood and urine of healthy athlete volunteers. Drug Test. Anal. 2019, 11, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A topical formulation of melatoninergic compounds exerts strong hypotensive and neuroprotective effects in a rat model of hypertensive glaucoma. Int. J. Mol. Sci. 2020, 21, 9267. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric Oxide (NO): An emerging target for the treatment of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated epigallocatechin-3-gallate (C18-EGCG) as novel lipophilic EGCG derivative: Characterization and antioxidant evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivieri, M.; Cristaldi, M.; Pezzino, S.; Lupo, G.; Anfuso, C.D.; Gagliano, C.; Genovese, C.; Rusciano, D. Experimental evidence of the healing properties of lactobionic acid for ocular surface disease. Cornea 2018, 37, 1058–1063. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnelli, C.; Piva, F.; Cecati, M.; Armeni, T.; Mobbili, G.; Galeazzi, R.; Melecchi, A.; Cristaldi, M.; Corsaro, R.; Rusciano, D. Meldonium Inhibits Cell Motility and Wound-Healing in Trabecular Meshwork Cells and Scleral Fibroblasts: Possible Applications in Glaucoma. Pharmaceuticals 2023, 16, 594. https://doi.org/10.3390/ph16040594
Minnelli C, Piva F, Cecati M, Armeni T, Mobbili G, Galeazzi R, Melecchi A, Cristaldi M, Corsaro R, Rusciano D. Meldonium Inhibits Cell Motility and Wound-Healing in Trabecular Meshwork Cells and Scleral Fibroblasts: Possible Applications in Glaucoma. Pharmaceuticals. 2023; 16(4):594. https://doi.org/10.3390/ph16040594
Chicago/Turabian StyleMinnelli, Cristina, Francesco Piva, Monia Cecati, Tatiana Armeni, Giovanna Mobbili, Roberta Galeazzi, Alberto Melecchi, Martina Cristaldi, Roberta Corsaro, and Dario Rusciano. 2023. "Meldonium Inhibits Cell Motility and Wound-Healing in Trabecular Meshwork Cells and Scleral Fibroblasts: Possible Applications in Glaucoma" Pharmaceuticals 16, no. 4: 594. https://doi.org/10.3390/ph16040594
APA StyleMinnelli, C., Piva, F., Cecati, M., Armeni, T., Mobbili, G., Galeazzi, R., Melecchi, A., Cristaldi, M., Corsaro, R., & Rusciano, D. (2023). Meldonium Inhibits Cell Motility and Wound-Healing in Trabecular Meshwork Cells and Scleral Fibroblasts: Possible Applications in Glaucoma. Pharmaceuticals, 16(4), 594. https://doi.org/10.3390/ph16040594