Effects of Water–Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties
Abstract
:1. Introduction
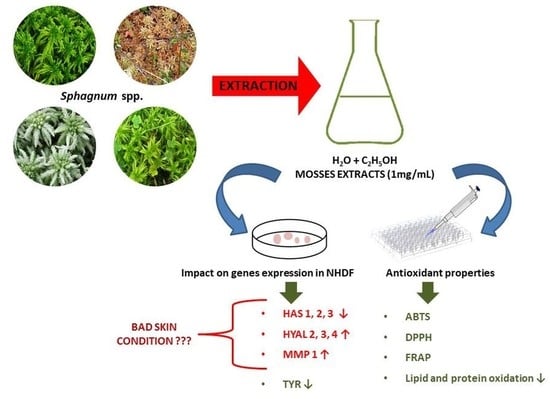
2. Results
2.1. Results of Determination of the Content of Phenolic Compounds
2.2. Results of the Antioxidant Properties of Moss Extracts
2.3. Results of the Cytotoxicity Study of Extracts from Four Sphagnum Species
2.4. Results of Genes Expression Determination
3. Discussion
4. Materials and Methods
4.1. Mosses and Extracts Preparation
4.2. Equipment, Materials and Reagents Used in the Determination of Phenolic Compounds and Antioxidant Activity
4.2.1. Equipment and Materials
4.2.2. Reagents
4.3. Cell line, Equipment, Materials and Reagents Used for Cell Culture and in Molecular Research
4.3.1. Equipment and Materials
4.3.2. Reagents
4.4. Determination of the Phenolic Compounds Content
4.4.1. TP Determination
4.4.2. TPA Determination
4.4.3. TF Determination
4.4.4. Determination of Selected Phenolic Compounds Using the HPLC Method
4.5. Determination of the Antioxidant Properties of Moss Extracts
4.5.1. DPPH Method
4.5.2. ABTS Method
4.5.3. FRAP Method
4.5.4. Study of the Impact on the Formation of AOPP In Vitro by Moss Extracts
4.5.5. Study of the Impact on PL by Moss Extracts
4.6. Study of the Influence of Moss Extracts on the Expression of Genes Encoding Selected Enzymes
4.6.1. Cell Culture Conditions NHDF
4.6.2. Molecular Analyses
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Parameter | Phenolic Compounds | |||
|---|---|---|---|---|
| p-Coumaric Acid | Rutin | Quercetin | Apigenin | |
| LOD (mg/L) | 0.25 | 0.32 | 0.21 | 0.12 |
| LOQ (mg/L) | 0.83 | 1.05 | 0.70 | 0.38 |
| Standard error (mg/L) | 0.08 | 0.10 | 0.07 | 0.04 |
| Linearity range (mg/L) | 0.83–100 | 1.05–100 | 0.70–100 | 0.38–100 |
References
- Oke, T.A.; Hager, H.A. Plant community dynamics and carbon sequestration in Sphagnum-dominated peatlands in the era of global change. Glob. Ecol. Biogeogr. 2020, 29, 1610–1620. [Google Scholar] [CrossRef]
- Shaw, A.J.; Cox, C.J.; Buck, W.R.; Devos, N.; Buchanan, A.M.; Cave, L.; Seppelt, R.; Shaw, B.; Larraín, J.; Andrus, R.; et al. Newly resolved relationships in an early land plant lineage: Bryophyta class Sphagnopsida (peat mosses). Am. J. Bot. 2010, 97, 1511–1531. [Google Scholar] [CrossRef] [Green Version]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Stebel, A. Peat Mosses of the ‘Lasy Środkowopomorskie’ Promotional Forest Complex (Poland, West Pomerania); Nadleśnictwo Karnieszewice: Trawica, Poland, 2017; p. 191. [Google Scholar]
- Rasmussen, S.; Wolff, C.; Rudolph, H. Compartmentalization of phenolic constituents in Sphagnum. Phytochemistry 1995, 38, 35–39. [Google Scholar] [CrossRef]
- Stalheim, T.; Ballance, S.; Christensen, B.E.; Granum, P.E. Sphagnan—A pectin-like polymer isolated from Sphagnum moss can inhibit the growth of some typical food spoilage and food poisoning bacteria by lowering the pH. J. Appl. Microbiol. 2009, 106, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Painter, T.J.; Christensen, B.E. Concerning the wound-healing properties of Sphagnum holocellulose: The Maillard reaction in pharmacology. J. Ethnopharmacol. 2003, 88, 145–148. [Google Scholar] [CrossRef]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.R.; Lee, D.; Eom, H.J.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Identification and mechanism of action of renoprotective constituents from peat moss Sphagnum palustre in cisplatin-induced nephrotoxicity. J. Funct. Foods 2016, 20, 358–368. [Google Scholar] [CrossRef]
- Nam, J.-H.; Jeong, J.-C.; Yoon, Y.-H.; Hong, S.-Y.; Kim, S.-J.; Jin, Y.-I.; Lee, Y.-J.; Yoo, D.-L.; Lee, K.-T.; Park, H.-J. Phytochemical constituents and anticancer activity of Sphagnum palustre extract. Korean J. Plant Resour. 2011, 24, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, G.; Portaluppi, M.C.; Salas, F.A.; Díaz, M.F. Biological properties of the Chilean native moss Sphagnum magellanicum. Biol. Res. 2009, 42, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.J.; Park, Y.J.; Kang, H.R.; Kim, H.R.; Bang, I.J.; Park, H.B.; Chung, K.H.; Kim, K.H. Inhibitory effect of Sphagnum palustre extract and its bioactive compounds on aromatase activity. Bangladesh J. Pharmacol. 2016, 11, 661–665. [Google Scholar] [CrossRef]
- Mellegård, H.; Stalheim, T.; Hormazabal, V.; Granum, P.E.; Hardy, S.P. Antibacterial activity of sphagnum acid and other phenolic compounds found in Sphagnum papillosum against food-borne bacteria. Lett. Appl. Microbiol. 2009, 49, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Fudyma, J.D.; Lyon, J.; AminiTabrizi, R.; Gieschen, H.; Chu, R.K.; Hoyt, D.W.; Kyle, J.E.; Toyoda, J.; Tolic, N.; Heyman, H.M.; et al. Untargeted metabolomic profiling of Sphagnum fallax reveals novel antimicrobial metabolites. Plant Direct 2019, 3, e00179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tienaho, J.; Silvan, N.; Muilu-Mäkelä, R.; Kilpeläinen, P.; Poikulainen, E.; Sarjala, T. Ultraviolet absorbance of Sphagnum magellanicum, S. fallax and S. fuscum extracts with seasonal and species-specific variation. Photochem. Photobiol. Sci. 2021, 20, 379–389. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Gallardo, C.; Puertas-Mejía, M.A. In vitro photoprotection and antioxidant capacity of Sphagnum meridense extracts, a novel source of natural sunscreen from the mountains of Colombia. Pure Appl. Chem. 2015, 87, 961–970. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, S.; Sharma, R.; Vats, S.; Nagaraju, G.P.; Alam, A. Phytochemical screening and antioxidant potential of Plagiochasma appendiculatum Lehm. & Lindenb. and Sphagnum fimbriatum Wilson. Plant Sci. Today 2022, 9, 986–990. [Google Scholar] [CrossRef]
- Benek, A.; Canli, K.; Altuner, E.M. Traditional medicinal uses of mosses. Anatol. Bryol. 2022, 8, 57–65. [Google Scholar] [CrossRef]
- Drobnik, J.; Stebel, A. Tangled history of the European uses of Sphagnum moss and sphagnol. J. Ethnopharmacol. 2017, 209, 41–49. [Google Scholar] [CrossRef]
- Opelt, K.; Berg, C.; Berg, G. The bryophyte genus Sphagnum is a reservoir for powerful and extraordinary antagonists and potentially facultative human pathogens. FEMS Microbiol. Ecol. 2007, 61, 38–53. [Google Scholar] [CrossRef]
- Dziwak, M.; Wróblewska, K.; Szumny, A.; Galek, R. Modern use of Bryophytes as a source of secondary metabolites. Agronomy 2022, 12, 1456. [Google Scholar] [CrossRef]
- Podterob, A.P.; Zubets, E.V. A history of the medicinal use of plants of the genus Sphagnum. Pharm. Chem. J. 2002, 36, 192–194. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol characterization and skin-preserving properties of hydroalcoholic flower extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, R.V.; Guillaumin, A.; Abdelwahab, A.B.; Salwinski, A.; Gotfredsen, C.H.; Bourgaud, F.; Enemark-rasmussen, K.; Miguel, S.; Simonsen, H.T. Collagenase and tyrosinase inhibitory effect of isolated constituents from the moss Polytrichum formosum. Plants 2021, 10, 1271. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Andrade, J.M.; Domínguez-Martín, E.M.; Nicolai, M.; Faustino, C.; Rodrigues, L.M.; Rijo, P. Screening the dermatological potential of Plectranthus species components: Antioxidant and inhibitory capacities over elastase, collagenase and tyrosinase. J. Enzym. Inhib. Med. Chem. 2021, 36, 257–269. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef] [Green Version]
- Motti, R.; Di Palma, A.; de Falco, B. Bryophytes used in folk medicine: An ethnobotanical overview. Horticulturae 2023, 9, 137. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Dey, A. The ethno-medicinal and pharmaceutical attributes of Bryophytes: A review. Phytomed. Plus 2022, 2, 100255. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of remedies, an ethno-medicinal review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Niederkrüger, H.; Busch, A.; Dabrowska-Schlepp, P.; Krieghoff, N.; Schaaf, A.; Frischmuth, T. Single-use processing as a safe and convenient way to develop and manufacture moss-derived biopharmaceuticals. In Single-Use Technology in Biopharmaceutical Manufacture; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 311–318. [Google Scholar] [CrossRef]
- Decker, E.L.; Reski, R. Mosses in biotechnology. Curr. Opin. Biotechnol. 2020, 61, 21–27. [Google Scholar] [CrossRef]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and anti-aging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Wang, P.; Wang, X. Skin ageing and cancer. In The Role of Matrix Metalloproteinase in Human Body Pathologies; InTech: Bellingham, WA, USA, 2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Smolińska-Kondla, D.; Zych, M.; Ramos, P.; Wacławek, S.; Stebel, A. Antioxidant potential of various extracts from 5 common European mosses and its correlation with phenolic compounds. Herba Pol. 2022, 68, 54–68. [Google Scholar] [CrossRef]
- Klavina, L.; Springe, G. Optimisation of conditions for extraction of biologically active compounds from common Bryophytes in Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2015, 69, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The antiglycoxidative ability of selected phenolic compounds—An in vitro study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical screening, antioxidant and sperm viability of Nelumbo nucifera petal extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant activity, total phenolic content and total flavonoid content in sweet chestnut (Castanea sativa Mill.) cultivars grown in Northwest Spain under different environmental conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S. Impact of drying methods on natural antioxidants, phenols and flavanones of immature dropped Citrus sinensis L. Osbeck fruits. Sci. Rep. 2022, 12, 6684. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Aida, W.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427–1435. [Google Scholar]
- Thoo, Y.Y.; Ho, K.S.; Liang, Y.J.; Ho, C.W.; Tan, P.C. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, antimicrobial and antioxidant activities of mosses obtained from open habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Villarroel, M.; Acevedo, C.; Yáñez, E.; Biolley, E. Functional properties of Sphagnum magellanicum fiber and its direct use in formulation of bakery products. Arch. Latinoam. Nutr. 2003, 53, 400–407. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Lunić, T.M.; Oalde, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Nedeljković, B.D.B. Extracts characterization and in vitro evaluation of potential immunomodulatory activities of the moss Hypnum cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gasowska-Bajger, B.; Wojtasek, H. Reactions of flavonoids with o-quinones interfere with the spectrophotometric assay of tyrosinase activity. J. Agric. Food Chem. 2016, 64, 5417–5427. [Google Scholar] [CrossRef] [PubMed]
- Wojtasek, H. Comment on “Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review” by R. Obaid, E. Mughal, N. Naeem, A. Sadiq, R.; Alsantali, R. Jassas, Z. Moussa and S. Ahmed, RSC Advances, 2021, 11, 22159. RSC Adv. 2022, 12, 5395–5397. [Google Scholar] [CrossRef] [PubMed]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.Y.; Kim, J. Synthesis and biological evaluation of the anti-melanogenesis effect of coumaric and caffeic acid-conjugated peptides in human melanocytes. Front. Pharmacol. 2020, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Tardif, G.; Reboul, P.; Pelletier, J.-P.; Martel-Pelletier, J. Ten years in the life of an enzyme: The story of the human MMP-13 (collagenase-3). Mod. Rheumatol. 2004, 14, 197–204. [Google Scholar] [CrossRef]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial regulation of matrix metalloproteinases for skin health. Enzyme Res. 2011, 2011, 427285. [Google Scholar] [CrossRef] [Green Version]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Nik Majid, N.M. Anti-elastase, anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef] [Green Version]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Avantaggiato, A.; Bertuzzi, G.; Vitiello, U.; Iannucci, G.; Pasin, M.; Pascali, M.; Cervelli, V.; Carinci, F. Role of antioxidants in dermal aging: An in vitro study by q-RT-PCR. Aesthetic Plast. Surg. 2014, 38, 1011–1016. [Google Scholar] [CrossRef]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Investig. Dermatol. 2001, 117, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tjermin, L.; Nyoman, I.; Lister, E.; Nasution, A.N.; Girsang, E. Antioxidant and inhibition of elastase effect of scutellarein and apigenin. Technol. Sci. Am. Sci. Res. J. Eng. 2019, 55, 104–110. [Google Scholar]
- Kavasi, R.M.; Berdiaki, A.; Spyridaki, I.; Corsini, E.; Tsatsakis, A.; Tzanakakis, G.; Nikitovic, D. HA metabolism in skin homeostasis and inflammatory disease. Food Chem. Toxicol. 2017, 101, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Gazzola, R.; Metalla, M.; Vaienti, L. The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthetic Surg. J. 2013, 33, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.J.; Wang, B.; Cui, Y.; Shi, M.; Zhao, Y.; Quan, T.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell Commun. Signal. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, Y.K.; Jung, J.-Y.; Shin, J.-E.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201. [Google Scholar] [CrossRef]
- Mihailović, V.; Kreft, S.; Benković, E.T.; Ivanović, N.; Stanković, M.S. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind. Crops Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Knoll, J.E. Estimation of the limit of detection in chromatography. J. Chromatogr. Sci. 1985, 23, 422–425. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzebyk, E.; Piwowar, A. The Tibetan herbal medicines Padma 28 and Padma Circosan inhibit the formation of advanced glycation endproducts (AGE) and advanced oxidation protein products (AOPP) in vitro. BMC Complement. Altern. Med. 2014, 14, 287. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]






| Species of Moss | Content of Determined Phenolic Compounds in mg/g of Dry Extract | |||
|---|---|---|---|---|
| p-Coumaric Acid | Rutin | Quercetin | Apigenin | |
| Sphagnum girgensohnii Russow. | 0.141 | 0.064 | - | - |
| Sphagnum magellanicum Brid. | 0.069 | 0.510 | 0.142 | - |
| Sphagnum palustre L. | 0.104 | 0.364 | - | - |
| Sphagnum squarrosum Crome | 0.263 | 0.102 | - | 0.053 |
| Moss Species Name | Photographs of Species | Morphological Features | Habitats |
|---|---|---|---|
| Sphagnum girgensohnii Russow. |  | Rather robust, green or yellowish-green plants, forming loose hummocks or mats | Wet forests and thicket communities, mid-forest peat bogs, |
| Sphagnum magellanicum Brid. |  | Robust plants, most often reddish to wine-red in colour, forming broad carpets or hummocks | Oligotrophic, most often raised and transitional bogs, marshy forests and birch forests, less often acidic low bogs |
| Sphagnum palustre L. |  | Robust plants, usually green, pale green, yellow-green, sometimes orange or brownish, forming loose carpets or tussocks | Swamp forests, low, transitional and raised bogs |
| Sphagnum squarrosum Crome |  | Large plants, with characteristically strongly bent branch leaves, from light green to yellow-green, in sunny places sometimes brownish, forming loose mats | Wet forests, thickets, stream banks, mid-forest springs and banks of water reservoirs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Urbisz, K.; Kimsa-Dudek, M.; Kamionka, M.; Dudek, S.; Raczak, B.K.; Wacławek, S.; Chmura, D.; Kaczmarczyk-Żebrowska, I.; Stebel, A. Effects of Water–Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties. Pharmaceuticals 2023, 16, 1076. https://doi.org/10.3390/ph16081076
Zych M, Urbisz K, Kimsa-Dudek M, Kamionka M, Dudek S, Raczak BK, Wacławek S, Chmura D, Kaczmarczyk-Żebrowska I, Stebel A. Effects of Water–Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties. Pharmaceuticals. 2023; 16(8):1076. https://doi.org/10.3390/ph16081076
Chicago/Turabian StyleZych, Maria, Katarzyna Urbisz, Magdalena Kimsa-Dudek, Maria Kamionka, Sławomir Dudek, Barbara Klaudia Raczak, Stanisław Wacławek, Damian Chmura, Ilona Kaczmarczyk-Żebrowska, and Adam Stebel. 2023. "Effects of Water–Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties" Pharmaceuticals 16, no. 8: 1076. https://doi.org/10.3390/ph16081076
APA StyleZych, M., Urbisz, K., Kimsa-Dudek, M., Kamionka, M., Dudek, S., Raczak, B. K., Wacławek, S., Chmura, D., Kaczmarczyk-Żebrowska, I., & Stebel, A. (2023). Effects of Water–Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties. Pharmaceuticals, 16(8), 1076. https://doi.org/10.3390/ph16081076