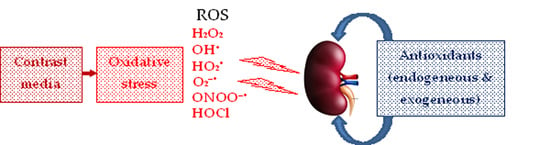
Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy)
Abstract
:1. Introduction
2. Contrast Media
3. Redox System
4. Antioxidants vs. Contrast-Induced Nephropathy
4.1. N-Acetyl-L-Cystein/Glutathione
4.2. L-Ascorbic Acid (Vitamin C)
4.3. Vitamin E (Tocopherols, Tocotrienols)
4.4. Bilirubin
4.5. Melatonin
4.6. L-Carnitine
4.7. Statins
4.8. Probucol
4.9. MESNA
4.10. Resveratrol
4.11. Carotenoids
4.12. Plant Antioxidants
4.12.1. Green Tea Extract
4.12.2. Grape Seed Proanthocyanidin Extract
4.12.3. Curcumin
4.13. Novel Antioxidant—Xylose–Pyrogallol Conjugate
4.14. Summary
5. Hybrid Contrast/Antioxidant Media as Theranostic Agents
5.1. Gd Complex/Rosmarinic Acid Conjugate
5.2. Nitroxyl Radicals
5.3. Theranostic Antioxidant Nanomaterials
5.3.1. Cerium Oxide Nanoparticles
5.3.2. Iron Oxide Nanoparticles
5.4. Summary
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellin, M.-F. MR contrast agents, the old and the new. Eur. J. Radiol. 2006, 60, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. BioMed Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paude, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodrígue, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Thomson, K.R.; Varma, D.K. Safe use of radiographic contrast media. Aust. Prescr. 2010, 33, 35–37. [Google Scholar] [CrossRef]
- Egbert, R.E.; De Cecco, C.N.; Schoep, U.J.; McQuiston, A.D.; Meinel, F.G.; Katzberg, R.W. Delayed adverse reactions to the parenteral administration of iodinated contrast media. Am. J. Roentgenol. 2014, 203, 1163–1170. [Google Scholar] [CrossRef]
- Kuşkonmaz, Ş.M.; Yıldız, S. Effect of iodinated contrast media on thyroid: A brief review. J. Health Sci. 2016, 6, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Rhee, C.M.; Leung, A.M.; Braverman, L.E.; Brent, G.A.; Pearce, E.N. A review: Radiographic iodinated contrast media induced thyroid dysfunction. J. Clin. Endocrinol. Metab. 2015, 100, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.J.; Yang, Z.L.; Zhang, L.J. Gadolinium deposition in brain: Current scientific evidence and future perspectives. Front. Mol. Neurosci. 2018, 11, 335. [Google Scholar] [CrossRef] [Green Version]
- Elserafy, A.S.; Abdelsalam, T. Contrast-induced nephropathy, 2020. In New Insight into Cerebrovascular Diseases—An Updated Comprehensive Review; Ambrosi, P.B., Ahmad, R., Abdullahi, A., Agrawal, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cho, E.; Ko, G.-J. The pathophysiology and the management of radiocontrast-induced nephropathy. Diagnostics 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Lass, N.; Glock, D. Renal hemodynamics in radiocontrast medium-induced renal dysfunction: A role for dopamine-1 receptors. Kidney Int. 1999, 56, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, T.G.; Bulugahapitiya, S. Contrast-induced nephropathy. Am. J. Roentgenol. 2004, 183, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Liss, P.; Hansell, P.; Carlsson, P.-O.; Fasching, A.; Palm, F. Iodinated contrast media decrease renomedullary blood flow. A possible cause of contrast media-induced nephropathy. Adv. Exp. Med. Biol. 2009, 645, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Faucon, A.L.; Bobrie, G.; Clement, O. Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. Eur. J. Radiol. 2019, 116, 231–241. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Z.; Wang, F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020, 259, 118379. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, H.; Hu, X.; Zhang, J.; Wu, Y.; Qiao, S.; Yang, Y.; Gao, R. A risk score model of contrast-induced acute kidney injury in patients with emergency percutaneous coronary interventions. Front. Cardiovasc. Med. 2022, 9, 989243. [Google Scholar] [CrossRef]
- Kusirisin, P.; Chattipakorn, S.C.; Chattipakorn, N. Contrast-induced nephropathy and oxidative stress: Mechanistic insights for better interventional approaches. J. Transl. Med. 2020, 18, 400. [Google Scholar] [CrossRef]
- Shams, E.; Mayrovitz, H.N. Contrast-induced nephropathy: A review of mechanisms and risks. Cureus 2021, 13, e14842. [Google Scholar] [CrossRef]
- Heyman, S.; Reichman, J.; Brezis, M. Pathophysiology of radiocontrast nephropathy: A role for medullary hypoxia. Investig. Radiol. 1999, 34, 685–691. [Google Scholar] [CrossRef]
- Brezis, M.; Rosen, S. Hypoxia of the renal medulla—Its implications for disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef]
- Katholi, R.E.; Woods, W.T.; Taylor, G.J.; Deitrick, C.L.; Womack, K.A.; Katholi, C.R.; McCann, W.P. Oxygen free radicals and contrast nephropathy. Am. J. Kidney Dis. 1998, 32, 64–71. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Liu, Z.; Bai, Y. Risk factors of contrast-induced nephropathy after percutaneous coronary intervention: A retrospective analysis. J. Int. Med. Res. 2021, 49, 3000605211005972. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ayoka, T.O.; Ezema, B.O.; Eze, C.N.; Nnadi, C.O. Antioxidants for the prevention and treatment of noncommunicable diseases. J. Explor. Res. Pharmacol. 2022, 7, 178–188. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; De Sarro, G.; Michael, A. The toxicity of iodinated radiographic contrast agents in the clinical practice. J. Nephrol. Adv. 2015, 1, 6–41. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, N.R.; Santos-Silva, A.; Coimbra, S.; Valente, M.J. Nephrotoxic mechanisms of gadolinium: Implications for the use of gadolinium-based contrast agents. Nephrol. Dial. Transplant. 2021, 36 (Suppl. 1), gfab079.004. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Goren, S.D.; Yudina, E.B.; Aleksenskii, A.E.; Vul’, A.Y.; Shames, A.I. Gd(III)-grafted detonation nanodiamonds for MRI contrast enhancement. J. Phys. Chem. C 2019, 123, 2627–2631. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Prager, O.; Swissa, E.; Kulvelis, Y.V.; Yudina, E.B.; Aleksenskii, A.E.; Goren, S.D.; Vul’, A.Y.; Shames, A.I. PVP-coated Gd-grafted nanodiamonds as a novel and potentially safer contrast agent for in vivo MRI. Magn. Reson. Med. 2021, 86, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Panich, A.M.; Shames, A.I.; Aleksenskii, A.E.; Yudina, E.B.; Vul’, A.Y. Manganese-grafted detonation nanodiamond, a novel potential MRI contrast agent. Diam. Relat. Mater. 2021, 119, 108590. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Aleksenskii, A.E.; Kulvelis, Y.V.; Chizhikova, A.; Vul’, A.Y.; Shames, A.I. Suspensions of manganese-grafted nanodiamonds: Preparation, NMR, and MRI study. Diam. Relat. Mater. 2023, 131, 109591. [Google Scholar] [CrossRef]
- Bórquez, D.A.; Urrutia, P.J.; Wilson, C.; van Zundert, B.; Núñez, M.T.; González-Billault, C. Dissecting the role of redox signaling in neuronal development. J. Neurochem. 2016, 137, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P. Principles in redox signaling: From chemistry to functional significance. Antioxid. Redox Signal. 2013, 18, 1557–1593. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The double-edged sword profile of redox signaling: Oxidative events as molecular switches in the balance between cell physiology and cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef]
- Zhang, S.X.; Sanders, E.; Fliesler, S.J.; Wang, J.J. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp. Eye Res. 2014, 125, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Devillier, P.; Durand, T.; Stanke-Labesque, F.; Bessard, G. Vascular biology of the isoprostanes. J. Vasc. Res. 2001, 38, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Connor, P.M.; Abe, M.; Cowley, A.W., Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 2007, 49, 1336–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive oxygen and nitrogen species–induced protein modifications: Implication in carcinogenesis and anticancer therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldéus, P.; Cotgreave, I.A. N-acetylcysteine. Methods Enzymol. 1994, 234, 482–492. [Google Scholar] [CrossRef]
- Sisombath, N.S.; Jalilehvand, F. Similarities between N-acetylcysteine and glutathione in binding to lead(II) ions. Chem. Res. Toxicol. 2015, 28, 2313–2324. [Google Scholar] [CrossRef] [Green Version]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Tepel, M.; van der Giet, M.; Schwarzfeld, C.; Laufer, U.; Liermann, D.; Zidek, W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N. Engl. J. Med. 2000, 343, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyu, K.-G.; Cheng, J.-J.; Kuan, P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J. Am. Coll. Cardiol. 2002, 40, 1383–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, J.; Chow, W.H.; Chan, T.M.; Lo, S.K.; Kwok, O.H.; Yip, A.; Fan, K.; Lee, C.H.; Lam, W.F. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: A randomized controlled trial. JAMA 2003, 289, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Sandoval, L.J.; Kosowsky, B.D.; Losordo, D.W. Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial). Am. J. Cardiol. 2002, 89, 356–358. [Google Scholar] [CrossRef]
- Baker, C.S.; Wragg, A.; Kumar, S.; De Palma, R.; Baker, L.R.; Knight, C.J. A rapid protocol for the prevention of contrast-induced renal dysfunction: The RAPPID study. J. Am. Coll. Cardiol. 2003, 41, 2114–2118. [Google Scholar] [CrossRef] [Green Version]
- Briguori, C.; Quintavalle, C.; De Micco, F.; Condorelli, G. Nephrotoxicity of contrast media and protective effects of acetylcysteine. Arch. Toxicol. 2011, 85, 165–173. [Google Scholar] [CrossRef]
- Liu, R.; Nair, D.; Ix, J.; Moore, D.H.; Bent, S. N-acetylcysteine for the prevention of contrast-induced nephropathy a systematic review and meta-analysis. J. Gen. Intern. Med. 2005, 20, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Biernacka-Fiałkowska, B.; Szuksztul, M.; Suślik, W.; Dzierwa, K.; Tekieli, Ł.; Kostkiewicz, M.; Podolec, P.; Pieniążek, P. Intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy—A prospective, single-center, randomized, placebo-controlled trial. The INPROC trial. Adv. Interv. Cardiol. 2018, 14, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Sharp, A.J.; Patel, N.; Reeves, B.C.; Angelini, G.D.; Fiorentino, F. Pharmacological interventions for the prevention of contrast-induced acute kidney injury in high-risk adult patients undergoing coronary angiography: A systematic review and meta-analysis of randomised controlled trials. Open Heart 2019, 6, e000864. [Google Scholar] [CrossRef] [Green Version]
- Boccalandro, F.; Amhad, M.; Smalling, R.W.; Sdringola, S. Oral acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Catheter. Cardiovasc. Interv. 2003, 58, 336–341. [Google Scholar] [CrossRef]
- Durham, J.D.; Caputo, C.; Dokko, J.; Zaharakis, T.; Pahlavan, M.; Keltz, J.; Dutka, P.; Marzo, K.; Maesaka, J.K.; Fishbane, S. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002, 62, 2202–2207. [Google Scholar] [CrossRef] [Green Version]
- Allaqaband, S.; Tumuluri, R.; Malik, A.M.; Gupta, A.; Volkert, P.; Shalev, Y.; Bajwa, T.K. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter. Cardiovasc. Interv. 2002, 57, 279–283. [Google Scholar] [CrossRef]
- Briguori, C.; Manganelli, F.; Scarpato, P.; Elia, P.P.; Golia, B.; Riviezzo, G.; Lepore, S.; Librera, M.; Villari, B.; Colombo, A.; et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J. Am. Coll. Cardiol. 2002, 40, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Sadat, U. N-acetylcysteine in contrast-induced acute kidney injury: Clinical use against principles of evidence based clinical medicine! Expert Rev. Cardiovasc. Ther. 2014, 12, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Weisbord, S.D.; Gallagher, M.; Jneid, H.; Garcia, S.; Cass, A.; Thwin, S.-S.; Conner, T.A.; Chertow, G.M.; Bhatt, D.L.; Shunk, K.; et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N. Engl. J. Med. 2018, 378, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Jin, E.-Z.; Yu, L.-H.; Li, Y.; Liu, N.-N.; Dong, Y.-M.; Li, X.; Li, X.-Q. Oral N-acetylcysteine for prophylaxis of contrast-induced nephropathy in patients following coronary angioplasty: A meta-analysis. Exp. Ther. Med. 2017, 14, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fu, Q.; Cao, L.; Jin, W.; Cheng, L.; Li, Z. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: A meta-analysis of randomized, controlled trials. PLoS ONE 2013, 8, e55124. [Google Scholar] [CrossRef] [Green Version]
- Penugonda, S.; Ercal, N. Comparative evaluation of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) on glutamate and lead-induced toxicity in CD-1 mice. Toxicol. Lett. 2011, 201, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Tao, A.; Bai, Y.; Deng, Y.; Chen, G. Effectiveness of N-acetylcysteine for the prevention of contrast-induced nephropathy: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016, 5, e003968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magner, K.; Ilin, J.V.; Clark, E.G.; Kong, J.W.Y.; Davis, A.; Hiremath, S. Meta-analytic techniques to assess the association between N-acetylcysteine and acute kidney injury after contrast administration. A systematic review and meta-analysis. JAMA Netw. Open. 2022, 5, e2220671. [Google Scholar] [CrossRef]
- Sůva, M.; Kala, P.; Poloczek, M.; Kaňovský, J.; Štípal, R.; Radvan, M.; Hlasensky, J.; Hudec, M.; Brázdil, V.; Řehořová, J. Contrast-induced acute kidney injury and its contemporary prevention. Front. Cardiovasc. Med. 2022, 9, 1073072. [Google Scholar] [CrossRef] [PubMed]
- Cepaityte, D.; Leivaditis, K.; Varouktsi, G.; Roumeliotis, A.; Roumeliotis, S.; Liakopoulos, V. N-Acetylcysteine: More than preventing contrast-induced nephropathy in uremic patients—Focus on the antioxidant and anti-inflammatory properties. Int. Urol. Nephrol. 2023, 55, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, H.; Coster, J.; Khalil, A.; Bot, J.; McCauley, R.D.; Hall, J.C. Glutathione. ANZ J. Surg. 2003, 73, 517–522. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.K.; Menezes-Benavente, L.; Galvão, V.C.; Margis-Pinheiro, M. Multigene families encode the major enzymes of antioxidant metabolism in Eucalyptus grandis L. Genet. Mol. Biol. 2005, 28, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, W.; Ma, S.; Lu, J.; Shi, H.; Ding, F. Reduced glutathione for prevention of renal outcomes in patients undergoing selective coronary angiography or intervention. J. Interv. Cardiol. 2015, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wu, B.W.; Zhang, J.J.; Luo, X.P.; Shi, H.M. Preventive effect of reduced glutathione on contrast-induced nephropathy in elderly patients undergoing coronary angiography or intervention: A randomized, controlled trial. Braz. J. Med. Biol. Res. 2015, 48, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, E.; Ren, S.; Gu, X.; Zheng, J.; Yang, J. Reduced glutathione does not further reduce contrast-induced nephropathy in elderly patients with diabetes receiving percutaneous coronary intervention. J. Int. Med. Res. 2020, 48, 0300060520964017. [Google Scholar] [CrossRef]
- Arrivi, A.; Truscelli, G.; Pucci, G.; Barillà, F.; Carnevale, R.; Nocella, C.; Sordi, M.; Dominici, M.; Tanzilli, G.; Mangieri, E. The combined treatment of glutathione sodium salt and ascorbic acid for preventing contrast-associated acute kidney injury in st-elevation myocardial infarction patients undergoing primary PCI: A hypothesis to be validated. Antioxidants 2023, 12, 773. [Google Scholar] [CrossRef]
- Mandl, J.; Szarka, A.; Bánhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernie, A.R.; Tohge, T. A cross-kingdom history. eLife 2015, 4, e07527. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Johnson, D.; Jarvis, S.M. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001, 18, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005, 25, 105–125. [Google Scholar] [CrossRef]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Hanashima, C.; Namiki, H. Reduced viability of vascular endothelial cells by high concentration of ascorbic acid in vitreous humor. Cell Biol. Int. 1999, 23, 287–298. [Google Scholar] [CrossRef]
- Ashino, H.; Shimamura, M.; Nakajima, H.; Dombou, M.; Kawanaka, S.; Oikawa, T.; Iwaguchi, T.; Kawashima, S. Novel function of ascorbic acid as an angiostatic factor. Angiogenesis 2003, 6, 259–269. [Google Scholar] [CrossRef]
- Ting, H.H.; Timimi, F.K.; Boles, K.S.; Creager, S.J.; Ganz, P.; Creager, M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non–insulin-dependent diabetes mellitus. J. Clin. Investig. 1996, 97, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Salvetti, A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998, 97, 2222–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat, U.; Usman, A.; Gillard, J.H.; Boyle, J.R. Does ascorbic acid protect against contrast-induced acute kidney injury in patients undergoing coronary angiography—A systematic review with meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2013, 62, 2167–2175. [Google Scholar] [CrossRef] [Green Version]
- Ong, R.Y.; Palanca, L.A.; Agustin, L. Vitamin C in the prevention of contrast-induced nephropathy among high-risk patients undergoing coronary angiogram: A meta-analysis. Asian J. Med. Health 2021, 19, 38–45. [Google Scholar] [CrossRef]
- Futuhi, F.; Malakootian, M.; Maleki, M.; Peighambari, M.M.; Moghadam, M.H.; Hosseini, M.J.; Boudagh, S.; Arabian, M. Intravenous vitamin C to prevent contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Iran. Heart J. 2022, 23, 149–159. [Google Scholar]
- Fotohi, F.; Arabian, M. MON-229 Intravenous vitamin C for the prevention of contrast-induced nephropathy in patients undergoing percutaneous coronary interventions. Kidney Int. Rep. 2019, 4, S395. [Google Scholar] [CrossRef]
- Romano, G.; Briguori, C.; Quintavalle, C.; Zanca, C.; Rivera, N.V.; Colombo, A.; Condorelli, G. Contrast agents and renal cell apoptosis. Eur. Heart J. 2008, 29, 2569–2576. [Google Scholar] [CrossRef]
- Boscheri, A.; Weinbrenner, C.; Botzek, B.; Reynen, K.; Kuhlisch, E.; Strasser, R.H. Failure of ascorbic acid to prevent contrast media induced nephropathy in patients with renal dysfunction. Clin. Nephrol. 2007, 68, 279–286. [Google Scholar] [CrossRef]
- Lee, H.-C.; Sheu, S.-H.; Liu, I.-H.; Lee, C.-C.; Hsieh, C.-C.; Yen, H.-W.; Lai, W.-T.; Chang, J.-G. Impact of short-duration administration of N-acetylcysteine, probucol and ascorbic acid on contrast-induced cytotoxicity. J. Nephrol. 2012, 25, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, H. Prevention of contrast-induced nephropathy with ascorbic acid. Intern. Med. 2012, 51, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, H.; Amini, S. Efficacy of ascorbic acid on reducing the development of contrast-induced nephropathy. Razavi Int. J. Med. 2017, 5, e37341. [Google Scholar] [CrossRef]
- Amr, A.M.I.M.; Asar, W.H.A.E.-H.; Kayed, M.O.F.; Allah, S.I.D.; Ramzy, A.A. The effect of administration of ascorbic acid in prevention of contrast induced nephropathy in patients with renal impairment undergoing coronary intervention. Al-Azhar Med. J. (Cardiol.) 2021, 50, 2045–2056. [Google Scholar] [CrossRef]
- Palli, E.; Makris, D.; Papanikolaou, J.; Garoufalis, G.; Tsilioni, I.; Zygoulis, P.; Zakynthinos, E. The impact of N-acetylcysteine and ascorbic acid in contrast-induced nephropathy in critical care patients: An open-label randomized controlled study. Crit. Care 2017, 21, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [Green Version]
- Wefers, H.; Sies, H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur. J. Biochem. 1988, 174, 353–357. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef]
- Ohrvall, M.; Sundlof, G.; Vessby, B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J. Intern. Med. 1996, 239, 111–117. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Cignarelli, A.; Pinto, S.; D’Onofrio, L.; Milluzzo, A.; Miccoli, R.; Penno, G.; Mannucci, E. Alpha-tocopherol and contrast-induced nephropathy: A meta-analysis of randomized controlled trials. Int. J. Vitam. Nutr. Res. 2019, 91, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Kim, S.N.; Park, H.W.; Chung, S.; Kim, K.S. Could vitamin E prevent contrast-induced acute kidney injury? A systematic review and meta-analysis. J. Korean Med. Sci. 2017, 32, 1468–1473. [Google Scholar] [CrossRef]
- Samadi, K.; Naghibi, M.; Shabestari, M.; Sharifipour, F.; Dalooee, M.K.; Raeesi, V.; Nik, S.M.; Samadi, M. Evaluation the effects of alpha-tocopherol in comparison with N-acetylcystein for prevention of contrast induced nephropathy (CIN) in CKD patients. Iran. J. Kidney Dis. 2020, 14, 26–30. [Google Scholar] [PubMed]
- Kitzler, T.M.; Jaberi, A.; Sendlhofer, G.; Rehak, P.; Binder, C.; Petnehazy, E.; Stacher, R.; Kotanko, P. Efficacy of vitamin E and N-acetylcysteine in the prevention of contrast induced kidney injury in patients with chronic kidney disease: A double blind, randomized controlled trial. Wien. Klin. Wochenschr. 2012, 124, 312–319. [Google Scholar] [CrossRef]
- Garby, L.; Noyes, W.D. Studies on hemoglobin metabolism. II. Pathways of hemoglobin iron metabolism in normal man. J. Clin. Investig. 1959, 38, 1484–1486. [Google Scholar] [CrossRef]
- Sticova, E.; Jirsa, M. New insights in bilirubin metabolism and their clinical implications. World J. Gastroenterol. 2013, 19, 6398–6407. [Google Scholar] [CrossRef]
- Adin, C.A.; Croker, B.P.; Agarwal, A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am. J. Physiol.-Ren. Physiol. 2005, 288, F778–F784. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Farese, J.; Baylis, C.; Croker, B.; Archer, L.; Adin, C. Hyperbilirubinemia’s protective effect against cisplatin nephrotoxicity in the Gunn rat. Anticancer Drugs 2008, 19, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Na, K.Y.; Chae, D.W.; Kim, Y.S.; Kim, S.; Chin, H.J. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku J. Exp. Med. 2010, 221, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.H.; Kim, S.S.; Kim, W.J.; Kim, J.H.; Nam, Y.J.; Park, S.B.; Jeon, Y.K.; Kim, B.H.; Kim, I.J.; Kim, Y.K. Low serum bilirubin level predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus. Korean J. Intern. Med. 2017, 32, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Hamamoto, S.; Kaneto, H.; Kamei, S.; Shimoda, M.; Tawaramoto, K.; Kanda-Kimura, Y.; Kawasaki, F.; Hashiramoto, M.; Matsuki, M.; Mune, T.; et al. Low bilirubin levels are an independent risk factor for diabetic retinopathy and nephropathy in Japanese patients with type 2 diabetes. Diabetes Metab. 2015, 41, 429–431. [Google Scholar] [CrossRef]
- Huang, S.-S.; Huang, P.-H.; Wu, T.-C.; Chen, J.-W.; Lin, S.-J. Association of serum bilirubin with contrast-induced nephropathy and future cardiovascular events in patients undergoing coronary intervention. PLoS ONE 2012, 7, e42594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuruşkan, E.; Saraçoğlu, E. Bilirubin levels are associated with contrast-induced nephropathy in peripheral artery disease. Angiology 2016, 68, 728–733. [Google Scholar] [CrossRef]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, G.L.; Barclay, R.C. Bilirubin as an antioxidant: Kinetic studies of the reaction of bilirubin with peroxyl radicals in solution, micelles, and lipid bilayers. Org. Lett. 2004, 6, 1539–1542. [Google Scholar] [CrossRef]
- Ziberna, L.; Martelanc, M.; Franko, M.; Passamonti, S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci. Rep. 2016, 6, 29240. [Google Scholar] [CrossRef] [Green Version]
- Doré, S.; Takahashi, M.; Ferris, C.D.; Zakhary, R.; Hester, L.D.; Guastella, D.; Snyder, S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 1999, 96, 2445–2450. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Snyder, S.H. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Lu, J.; Xiong, W.; Oger, J.; Tetzlaff, W.; Cynader, M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2008, 181, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Otero-Regino, W.; Velasco, H.; Sandoval, H. The protective role of bilirubin in human beings. Rev. Colomb. Gastroenterol. 2009, 24, 293–301. [Google Scholar]
- Tsai, M.-T.; Tarng, D.-C. Beyond a measure of liver function—Bilirubin acts as a potential cardiovascular protector in chronic kidney disease patient. Int. J. Mol. Sci. 2019, 20, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Park, S.C. Physiological antioxidative network of the bilirubin system in aging an age-related diseases. Front. Pharmacol. 2012, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Nath, M.; Agarwal, A. New insights into the role of heme oxygenase-1 in acute kidney injury. Kidney Res. Clin. Pract. 2020, 39, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.; Bulmer, A.C.; Coombes, J.S.; Fassett, R.G. Circulating bilirubin and defense against kidney disease and cardiovascular mortality: Mechanisms contributing to protection in clinical investigations. Am. J. Physiol.-Ren. Physiol. 2014, 307, F123–F136. [Google Scholar] [CrossRef] [Green Version]
- Luo, E.; Wang, D.; Qiao, Y.; Zhu, B.; Liu, B.; Hou, J.; Tang, C. Association of total bilirubin with contrast-induced nephropathy in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Coron. Artery Dis. 2020, 31, 92–94. [Google Scholar] [CrossRef]
- Fonseca, C.; Watanabe, M.; Couto, S.; Santos, A.; Borges, F.; Vattimo, M. The renoprotective effects of Heme Oxygenase-1 during contrast-induced acute kidney injury in preclinical diabetic models. Clinics 2021, 76, e3002. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Devesa, I. Inducers of Heme Oxygenase-1. Curr. Pharm. Des. 2008, 14, 473–486. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived Heme-Oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Soto Conti, C.P. Bilirubin: The toxic mechanisms of an antioxidant molecule. Arch. Argent. Pediatr. 2021, 119, e18–e25. [Google Scholar] [CrossRef] [PubMed]
- Özgüner, M.F.; Serel, T.A.; Delibaş, N.; Tahan, V.; Koyu, A.; Çalişkan, S.; Köylü, H. The effect of melatonin on shock wave induced renal damage. East. J. Med. 1998, 3, 48–50. [Google Scholar]
- Zhang, C.; Suo, M.; Liu, L.; Qi, Y.; Zhang, C.; Xie, L.; Zheng, X.; Ma, C.; Li, J.; Yang, J.; et al. Melatonin alleviates contrast-induced acute kidney injury by activation of Sirt3. Oxid. Med. Cell. Longev. 2021, 2021, 6668887. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Gitto, E.; Sainz, R.M.; Mayo, J.C.; Leon, J.; Manchester, L.C.; Vijayalaxmi, A.; Kilic, E.; Kilic, Ü. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol. J. Pharmacol. 2004, 56, 159–170. [Google Scholar]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective role of melatonin and its metabolites in skin aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Markowska, M.; Niemczyk, S.; Romejko, K. Melatonin treatment in kidney diseases. Cells 2023, 12, 838. [Google Scholar] [CrossRef]
- Reiter, R.J. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: A brief review. Braz. J. Med. Biol. Res. 1993, 26, 1141–1155. [Google Scholar]
- Shida, C.S.; Castrucci, A.M.L.; Lamy-Freund, M.T. High melatonin solubility in aqueous medium. J. Pineal Res. 1994, 16, 198–201. [Google Scholar] [CrossRef]
- Gazi, S.; Altun, A.; Erdogan, O. Contrast-induced nephropathy: Preventive and protective effects of melatonin. J. Pineal Res. 2006, 41, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Bayır, A.; Kara, H.; Kıyıcı, A.; Kıyıcı, H.; Ak, A. The effects of melatonin on oxidative stress markers in an animal model of radiocontrast-induced nephropathy. Biomed. Res. 2011, 22, 311–318. [Google Scholar]
- Nasri, H.; Tavakoli, M.; Ahmadi, A.; Baradaran, A.; Nematbakhsh, M.; Rafieian-Kopaei, M. Ameliorative effect of melatonin against contrast media induced renal tubular cell injury. Pak. J. Med. Sci. 2014, 30, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shawadfy, M.G.; Kamel, G.A.M.; Abd-Allah, A.R.A. Crosstalk among apoptosis, inflammation, and autophagy in relation to melatonin protective effect against contrast-induced nephropathy in rats. Can. J. Physiol. Pharmacol. 2022, 100, 858–867. [Google Scholar] [CrossRef]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41 (Suppl. 4), S4–S12. [Google Scholar] [CrossRef]
- Brevetti, G.; Perna, S. Metabolic and clinical effects of L-carnitine in peripheral vascular disease. In L-Carnitine and Its Role in Medicine: From Function to Therapy; Ferrari, R., DiMauro, S., Sherwood, G., Eds.; Academic Press: London, UK, 1992; pp. 359–378. [Google Scholar]
- Calò, L.A.; Pagnin, E.; Davis, P.A.; Semplicini, A.; Nicolai, R.; Calvani, M.; Pessina, A.C. Antioxidant effect of L-carnitine and its short chain esters: Relevance for the protection from oxidative stress related cardiovascular damage. Int. J. Cardiol. 2006, 107, 54–60. [Google Scholar] [CrossRef]
- Gülcin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Jafari, A.; Dashti-Khavidaki, S.; Khalili, H.; Lessan-Pezeshki, M. Potential nephroprotective effects of L-carnitine against drug-induced nephropathy: A review of literature. Expert Opin. Drug Saf. 2013, 12, 523–543. [Google Scholar] [CrossRef]
- Ulinski, T.; Cirulli, M.; Virmani, M.A. The role of L-carnitine in kidney disease and related metabolic dysfunctions. Kidney Dial. 2023, 3, 178–191. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hajhossein Talasaz, A.; Alidoosti, M.; Pour Hosseini, H.R.; Gholami, K.; Jalali, A.; Aryannejad, H. Nephroprotective effects of L-carnitine against contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: A randomized open-labelled clinical trial. J. Tehran Heart Cent. 2017, 12, 57–64. [Google Scholar] [PubMed]
- Ramezanzade, E.; Nikfarjam, S.; Jafari, A.; Sanchooli, A.; Salari, A.; Ashouri, A. An investigation into how L-carnitine consumption prevents contrast-induced nephropathy: A case study of patients getting coronary angiograms in Iran. Res. Sq. 2023, 1–14. [Google Scholar] [CrossRef]
- Kavalipati, N.; Shah, J.; Ramakrishan, A.; Vasnawala, H. Pleiotropic effects of statins. Indian J. Endocrinol. Metab. 2015, 19, 554–562. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.A. Pleiotropic effects of statins. Curr. Atheroscler. Rep. 2000, 2, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Kawashima, S.; Takeshita, S.; Yamashita, T.; Azumi, H.; Yasuhara, M.; Nishi, H.; Inoue, N.; Yokoyama, M. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001, 154, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, M.; Toso, A.; Maioli, M.; Tropeano, F.; Villani, S.; Bellandi, F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J. Am. Coll. Cardiol. 2014, 63, 71–79. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, G.; Han, L.; Hou, F.; Huang, W.; Liu, H.; Gan, J.; Jiang, T.; Li, X.; Wang, W.; et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J. Am. Coll. Cardiol. 2014, 63, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Qiao, B.; Deng, J.; Li, Y.; Wang, X.; Han, Y. Rosuvastatin attenuated contrast-induced nephropathy in diabetes patients with renal dysfunction. Int. J. Clin. Exp. Med. 2015, 8, 2342–2349. [Google Scholar]
- Hammami, R.; Masmoudi, O.; Jdidi, J.; Turki, M.; Charfi, R.; Ben Mrad, I.; Bahloul, A.; Ellouze, T.; Gargouri, R.; Kammoun, S.; et al. Impact of atorvastatin reload on the prevention of contrast induced nephropathy in patients on chronic statin therapy: A prospective randomized trial. PLoS ONE 2023, 18, e0270000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Liu, C.; Li, R.; Zou, M.; Cheng, G. Efficacy of short-term statin treatment for the prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography/percutaneous coronary intervention: A meta-analysis of 21 randomized controlled trials. Am. J. Cardiovasc. Drugs 2016, 16, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dai, J.; Xu, X.; Wang, Z.; Xu, H.; Chen, J.; Qiu, Y.; Mao, W. Comparative efficacy of statins for prevention of contrast-induced acute kidney injury in patients with chronic kidney disease: A network meta-analysis. Angiology 2019, 70, 305–316. [Google Scholar] [CrossRef]
- Cho, A.; Lee, Y.K.; Sohn, S.Y. Beneficial effect of statin on preventing contrast-induced acute kidney injury in patients with renal insufficiency: A meta-analysis. Medicine 2020, 99, e19473. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Khan, M.; Rahman, H.; Khan, M.; Riaz, H.; Novak, M.; Opoku-Asare, I.; Kaluski, E. Bayesian network meta-analysis of preventive strategies for contrast-induced nephropathy after cardiac catheterization. Cardiovasc. Revasc. Med. 2019, 20, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, G.; Yang, C.; Li, Y.; Jing, Q.; Han, Y. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. J. Transl. Med. 2015, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Al-Otaibi, K.; Al Elaiwi, A.; Tariq, M.; Al-Asmari, A. Simvastatin attenuates contrast-induced nephropathy through modulation of oxidative stress, proinflammatory myeloperoxidase, and nitric oxide. Oxid. Med. Cell. Longev. 2012, 2012, 831748. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, S.; Yaylak, B.; Altay, S.; Kasikcioglu, H.; Cam, N. The role of statins in preventing contrast-induced acute kidney injury: A narrative review. Angiology 2015, 66, 701–707. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xu, X.; Ge, W.; Yang, S.; Lu, C. Effects of probucol on contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Medicine 2019, 98, e16049. [Google Scholar] [CrossRef]
- Ma, X.; Jiao, Z.; Liu, Y.; Chen, J.; Li, G.; Liu, T.; Tse, G.; Yuan, R. Probucol protects against contrast-induced acute kidney injury via the extracellular signal-regulated kinases 1 and 2 (ERK1/2)/JNK-caspase 3 pathway in diabetic rats. Med. Sci. Monit. 2019, 25, 1038–1045. [Google Scholar] [CrossRef]
- Xin, W.; Lin, Z.; Zhang, T.; Jia, S. Probucol for the prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography or percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Clin. Nephrol. 2019, 92, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Vania, R.; Lukito, A.A. The role of probucol preventing contrast-induced nephropathy in patients undergoing invasive coronary procedures—Systematic review and meta-analysis of randomized controlled trials. Turk. Kardiyol. Dern. Ars. 2021, 49, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xie, B.; Wang, H.; Liu, F.; Mei, L.; Qin, F.; Zhang, J.; Yi, X. Preventing contrast-induced acute kidney injury with probucol and hydration in patients with coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. Medicine 2023, 102, e33273. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, L.; Michoudet, C.; Cochat, P.; Baverel, G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J. Am. Soc. Nephrol. 2001, 12, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Gressier, B.; Lebegue, N.; Brunet, C.; Luyckx, M.; Dine, T.; Cazin, M.; Cazin, J.C. Scavenging of reactive oxygen species by letosteine, a molecule with two blocked SH groups. Comparison with free SH drugs. Pharm. World Sci. 1995, 17, 76–80. [Google Scholar] [CrossRef]
- Kabasakal, L.; Sehirli, A.O.; Cetinel, S.; Cikler, E.; Gedik, N.; Sener, G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004, 75, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Sehirli, O.; Ercan, F.; Sirvanci, S.; Gedik, N.; Kacmaz, A. Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg. Today 2005, 35, 575–580. [Google Scholar] [CrossRef]
- Haeussler, U.; Riedel, M.; Keller, F. Free reactive oxygen species and nephrotoxicity of contrast agents. Kidney Blood Press. Res. 2004, 27, 167–171. [Google Scholar] [CrossRef]
- Mashiach, E.; Sela, S.; Weinstein, T.; Cohen, H.I.; Shasha, S.M.; Kristal, B. Mesna: A novel reno-protective antioxidant in ischaemic acute renal failure. Nephrol. Dial. Transplant. 2001, 16, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, U.; Riedel, M.K.; Backes, M.; Imhof, A.; Muche, R.; Keller, F. MESNA (sodium 2-mercaptoethanesulfonate) for prevention of contrast medium-induced nephrotoxicity—Controlled trial. Clin. Nephrol. 2011, 75, 302–308. [Google Scholar] [CrossRef]
- Stilbenes-Resveratrol in Foods and Beverages, Version 3.6. Phenol-Explorer. 2016. Available online: http://phenol-explorer.eu/contents/polyphenol/592 (accessed on 13 May 2016).
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.A.; Bae, S.Y.; Ahn, S.Y.; Kim, J.; Kwon, Y.J.; Jung, W.Y.; Ko, G.J. Resveratrol ameliorates contrast induced nephropathy through the activation of SIRT1-PGC-1α-Foxo1 signaling in mice. Kidney Blood Press. Res. 2017, 42, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Qi, X.; Zhang, X.; Ren, K. Resveratrol protects against post-contrast acute kidney injury in rabbits with diabetic nephropathy. Front. Pharmacol. 2019, 10, 833. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.T.; Chen, Y.Y.; Lai, Y.H.; Cheng, C.C.; Lin, T.C.; Su, Y.S.; Liu, C.H.; Lai, P.C. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Int. J. Molecular Med. 2016, 37, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Cheng, C.C.; Lin, T.C.; Huang. Resveratrol ameliorates apoptosis induced by contrast medium ioxitalamate in HK-2 human renal proximal tubule cells in vitro. Crit. Care 2014, 18, P383. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Fu, Y.-C.; Wu, M.-J. Does resveratrol play a role in decreasing the inflammation associated with contrast induced nephropathy in rat model? J. Clin. Med. 2019, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Fragkiadoulaki, E.; Tsatsakis, A.; Nikitovic, D.; Georgiadis, G.; Kalogeraki, A.; Kaloudis, K.; Alegkakis, A.; Karzi, V.; Mamoulakis, C. Resveratrol and lycopene ameliorate contrast-induced nephropathy in a rabbit model. Hum. Exp. Toxicol. 2022, 41, 09603271221145355. [Google Scholar] [CrossRef]
- Carotenoids; Micronutrient Information Center, Linus Pauling Institute, Oregon State University, 1 August 2016. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/carotenoids (accessed on 17 April 2019).
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Zhou, H.L.; Huang, C.L.; You, C.-G.; Fang, Q.; Wu, P.; Wang, X.-G.; Han, C.-M. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 2015, 13, 2105–2123. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Fu, K.; Zhao, X.; Zhang, Y.; Yuan, Y.; Zhang, S.; Gu, X.; Guo, H. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J. Transl. Med. 2015, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Mosaad, Y.O.; Gobba, N.A.; Hussein, M.A. Astaxanthin; a promising protector against gentamicin-induced nephrotoxicity in rats. Curr. Pharm. Biotechnol. 2016, 17, 1189–1197. [Google Scholar] [CrossRef]
- Gao, D.; Li, W. Research progress of astaxanthin on contrast agent induced acute kidney injury. J. Cardiol. Cardiovasc. Sci. 2018, 2, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palabiyik, S.S.; Erkekoglu, P.; Zeybek, N.D.; Kizilgun, M.; Baydar, D.E.; Sahin, G.; Giray, B.K. Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp. Toxicol. Pathol. 2013, 65, 853–861. [Google Scholar] [CrossRef]
- Bedir, F.; Kocaturk, H.; Turangezli, O.; Sener, E.; Akyuz, S.; Ozgeris, F.B.; Dabanlioglu, B.; Suleyman, H.; Altuner, D.; Suleyman, B. The protective effect of lycopene against oxidative kidney damage associated with combined use of isoniazid and rifampicin in rats. Braz. J. Med. Biol. Res. 2021, 54, e10660. [Google Scholar] [CrossRef]
- Buyuklu, M.; Kandemir, F.M.; Ozkaraca, M.; Set, T.; Bakirci, E.M.; Topal, E.; Ileriturk, M.; Turkmen, K. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum. Exp. Toxicol. 2015, 34, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Hosseinzadeh, H. Preventing contrast-induced nephropathy (CIN) with herbal medicines: A review. Phytother. Res. 2021, 35, 1130–1146. [Google Scholar] [CrossRef]
- Rafieian-kopaei, M.; Ghasemi, Z. Plants used to decrease serum creatinine levels and contrast-induced nephropathy: A review article. Future Nat. Prod. 2021, 7, 48–65. Available online: http://futurenatprod.skums.ac.ir/article_247484.html (accessed on 9 June 2023).
- Yokozawa, T.; Noh, J.S.; Park, C.H. Green tea polyphenol protection against renal damage caused by oxidative stress. Evid.-Based Complement. Altern. Med. 2012, 2012, 845917. [Google Scholar] [CrossRef]
- Vaz, S.R.; de Amorim, L.M.N.; de Nascimento, P.V.F.; Veloso, V.S.P.; Nogueira, M.S.; Castro, I.A.; Mota, J.F.; Botelho, P.B. Effects of green tea extract on oxidative stress and renal function in diabetic individuals: A randomized, double-blinded, controlled trial. J. Funct. Foods 2018, 46, 195–201. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Ranjbar, A.; Hosseini, A.; Khanavi, M. The effect of green tea extract on oxidative stress and spatial learning in streptozotocin-diabetic rats. Iran. J. Pharm. Res. 2017, 16, 201–209. [Google Scholar] [PubMed]
- Tian, J.; Geiss, C.; Zarse, K.; Madreiter-Sokolowski, C.T.; Ristow, M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging (Albany NY) 2021, 13, 22629–22648. [Google Scholar] [CrossRef]
- Asadi, S.Y.; Parsaei, P.; Karimi, M.; Ezzati, S.; Zamiri, A.; Mohammadizadeh, F.; Rafieian-Kopaei, M. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int. J. Surg. 2013, 11, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Nasri, H.; Ahmadi, A.; Baradaran, A.; Nasri, P.; Hajian, S.; Pour-Arian, A.; Kohi, G.; Rafieian-Kopaei, M. A biochemical study on ameliorative effect of green tea (Camellia sinensis) extract against contrast media induced acute kidney injury. J. Ren. Inj. Prev. 2014, 3, 47–49. [Google Scholar] [CrossRef]
- Nasri, H.; Hajian, S.; Ahmadi, A.; Baradaran, A.; Kohi, G.; Nasri, P.; Rafieian-Kopaei, M. Ameliorative effect of green tea against contrast-induced renal tubular cell injury. Iran. J. Kidney Dis. 2015, 9, 421–426. [Google Scholar] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef]
- Bagchi, D.; Sen, C.K.; Ray, S.D.; Das, D.K.; Bagchi, M.; Preuss, H.G.; Vinson, J.A. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. 2003, 523, 87–97. [Google Scholar] [CrossRef]
- Ozkan, G.; Ulusoy, S.; Orem, A.; Ersoz, S.; Alkanat, M.; Yucesan, F.B.; Kaynar, K.; Al, S. Protective effect of the grape seed proanthocyanidin extract in a rat model of contrast-induced nephropathy. Kidney Blood Press. Res. 2012, 35, 445–453. [Google Scholar] [CrossRef]
- Ulusoy, S.; Ozkan, G.; Mungan, S.; Orem, A.; Yulug, E.; Alkanat, M.; Yucesan, F.B. GSPE is superior to NAC in the prevention of contrast-induced nephropathy: Might this superiority be related to caspase 1 and calpain 1? Life Sci. 2014, 103, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, B.; Huang, L.; Ding, H.; Huang, W. Curcumin attenuates contrast-induced nephropathy by upregulating heme oxygenase-1 expression in rat. Zhonghua Xin Xue Guan Bing Za Zhi 2013, 41, 116–120. [Google Scholar]
- Buyuklu, M.; Kandemir, F.M.; Ozkaraca, M.; Set, T.; Bakirci, E.M.; Topal, E. Protective effect of curcumin against contrast induced nephropathy in rat kidney: What is happening to oxidative stress, inflammation, autophagy and apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 461–470. [Google Scholar]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Huang, S.; Tang, Y.; Liu, T.; Zhang, N.; Yang, X.; Yang, D.; Hong, G. A Novel antioxidant protects against contrast medium-induced acute kidney injury in rats. Front. Pharmacol. 2020, 11, 599577. [Google Scholar] [CrossRef]
- Xiang, Y.; Ji, M.; Wu, L.; Lv, L.; Liang, Q.; Deng, R.; Deng, Z.; Liu, X.; Ren, L.; Feng, X.; et al. Rosmarinic acid prevents cisplatin-induced liver and kidney injury by inhibiting inflammatory responses and enhancing total antioxidant capacity, thereby activating the Nrf2 signaling pathway. Molecules 2022, 27, 7815. [Google Scholar] [CrossRef]
- Kim, H.-K.; Hwang, S.; Sung, B.; Kim, Y.-H.; Chang, Y. Gd-complex of a rosmarinic acid conjugate as an anti-inflammatory theranostic agent via reactive oxygen species scavenging. Antioxidants 2020, 9, 744. [Google Scholar] [CrossRef]
- Griesser, M.; Shah, R.; Van Kessel, A.T.; Zilka, O.; Haidasz, E.A.; Pratt, D.A. The catalytic reaction of nitroxides with peroxyl radicals and its relevance to their cytoprotective properties. J. Am. Chem. Soc. 2018, 140, 3798–3808. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakanishi, I.; Zhelev, Z.; Bakalova, R.; Aoki, I. Nitroxyl radical as a theranostic contrast agent in magnetic resonance redox imaging. Antioxid. Redox Signal. 2022, 36, 95–121. [Google Scholar] [CrossRef]
- Zhelev, Z.; Bakalova, R.; Aoki, I.; Matsumoto, K.; Gadjeva, V.; Anzai, K.; Kanno, I. Nitroxyl radicals as low toxic spin-labels for non-invasive magnetic resonance imaging of blood–brain barrier permeability for conventional therapeutics. Chem. Commun. 2009, 45, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yakumaru, H.; Narazaki, M.; Nakagawa, H.; Anzai, K.; Ikehira, H.; Ikota, N. Modification of nitroxyl contrast agents with multiple spins and their proton T1 relaxivity. Magn. Reson. Imaging 2008, 26, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kleschyov, A.L.; Sen’, V.; Golubev, V.; Münnemann, K.; Hinderberger, D.; Lackner, K.J.; Weber, S.; Terekhov, M.; Schreiber, L.M.; Münzel, T. Heparin-polynitroxides: Synthesis and preliminary evaluation as cardiovascular EPR/MR imaging probes and extracellular space-targeted antioxidants. Eur. J. Med. Chem. 2012, 58, 265–271. [Google Scholar] [CrossRef]
- Sen, V.D.; Sokolova, E.M.; Neshev, N.I.; Kulikov, A.V.; Pliss, E.M. Low molecular chitosan–(poly)nitroxides: Synthesis and evaluation as antioxidants on free radical-induced erythrocyte hemolysis. React. Funct. Polym. 2017, 111, 53–59. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Gupta, A.; Das, S.; Neal, C.J.; Seal, S. Controlling the surface chemistry of cerium oxide nanoparticles for biological applications. J. Mater. Chem. B 2016, 4, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.; Truong, A.H.T.; Brommesson, C.; du Rietz, A.; Kokil, G.R.; Boyd, R.D.; Hu, Z.; Dang, T.T.; Persson, P.O.A.; Uvdal, K. Cerium oxide nanoparticles with entrapped gadolinium for high T1 relaxivity and ROS-scavenging purposes. ACS Omega 2022, 7, 21337–21345. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.L.; Abakumov, M.A.; Savintseva, I.V.; Ermakov, A.M.; Popova, N.R.; Ivanova, O.S.; Kolmanovich, D.D.; Baranchikov, A.E.; Ivanov, V.K. Biocompatible dextran-coated gadolinium-doped cerium oxide nanoparticles as MRI contrast agents with high T1 relaxivity and selective cytotoxicity to cancer cells. J. Mater. Chem. B 2021, 9, 6586–6599. [Google Scholar] [CrossRef]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- O’Shea, A.; Parakh, A.; Lahoud, R.M.; Hedgire, S.; Harisinghani, M.G. The evolution of iron oxide nanoparticles as MRI contrast agents. MRS Adv. 2020, 5, 2157–2168. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic nanoparticles as MRI contrast agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New frontiers in molecular imaging with superparamagnetic iron oxide nanoparticles (SPIONs): Efficacy, toxicity, and future applications. Nucl. Med. Mol. Imaging 2020, 54, 65–80. [Google Scholar] [CrossRef]
- Shah, S.T.; Yehye, W.A.; Chowdhury, Z.Z.; Simarani, K. Magnetically directed antioxidant and antimicrobial agent: Synthesis and surface functionalization of magnetite with quercetin. Peer J. 2019, 7, e7651. [Google Scholar] [CrossRef] [Green Version]
- Rezayan, A.H.; Kheirjou, S.; Edrisi, M.; Ardestani, M.S.; Alvandi, H. A modified PEG-Fe3O4 magnetic nanoparticles conjugated with D(+) glucosamine (DG): MRI contrast agent. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1988–1998. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic nanoparticles for biomedical applications: From the soul of the earth to the deep history of ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging: Fundamentals, challenges, applications, and prospectives. Adv. Mater. 2020, 33, 1906539. [Google Scholar] [CrossRef]














| Antioxidant | Results of Using against CIN | Clinical Trials (Studies) |
|---|---|---|
| NAC | positive | on humans [53,54,55,56,57,58,60], meta-analyses [59,61,71] |
| no results | on humans [62,63,64,65,66,67], meta-analyses [68,69,72] | |
| Glutathione | no results | on humans [78,79,80] |
| Vitamin C | positive | on humans [96,97,98], meta-analyses [94,95] |
| no results | on humans [99,100,101,102,103,104] | |
| Vitamin E | positive | on humans, meta-analyses [113,114] |
| no results | on humans [115,116] | |
| Bilirubin/HO-1 | positive (expected) | Association of bilirubin level with CIN was found in [124,125,139]; induction of HO-1 (on animals) [140] |
| Melatonin | positive | on animal models [146,149,152,153,154,155] |
| L-carnitine | positive | on humans [162,163,164,165] |
| Statins | positive | on humans [170,171,172,173], meta-analyses [174,175,176,177], on animals [178,179] |
| controversial | on humans [180] | |
| Probucol | positive | on humans [181,182], meta-analyses [183,185] |
| no results | on humans, meta-analysis [184] | |
| MESNA | positive | on humans [190,192] |
| Resveratrol | positive | on animal models [195,196], in vitro [197,198,199,200] |
| Astaxanthin | positive | on animal models [204,205,206,207] |
| Lycopene | positive | on animal models [200,209,210,211] |
| Green tea extract | positive | on animal models [219,220] |
| GSPE | positive | on animal models [224,225] |
| Curcumin | positive | on animal models [228,229] |
| Xylose-pyrogallol conjugate | positive | on animal models [231] |
| Hybrid Medium | Studies |
|---|---|
| Gd complex/RosA conjugate | in vitro and on animal model (antioxidant and MRI) [233] |
| Nitroxyl radicals | MRI, EPR, redox properties [235,237] |
| Nitroxyl–Lomustine (anticancer) | MRI, blood–brain barrier permeability [236] |
| Nitroxyl radicals–heparin | in vitro and on animal model (antioxidant and MR properties) [238] |
| Nitroxyl radicals–chitosan | in vitro (antioxidant properties, EPR) [239] |
| Cerium oxide nanoparticles doped with Gd | dynamic light scattering, Zeta potential measurement, X-ray diffraction, high-resolution transmission electron microscopy, near edge X-ray absorption fine structure, MRI properties, antioxidant properties in vitro [244,245] For the nanoparticles coated with dextran—cancer cytotoxicity [246] |
| Functionalized by quercetin magnetite nanoparticles | antioxidant and antibacterial properties in vitro, FTIR, Raman, TEM, X-ray spectroscopies, magnetic properties [251] |
| Functionalized by PEG + D(+) glucosamine magnetite nanoparticles | XRD, VSM, FESEM, and FTIR analyses; MRI properties, particle size, zeta potential, biodistribution analysis, very slight kidney cytotoxicity [252] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, I.G.; Tatikolov, A.S. Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy). Pharmaceuticals 2023, 16, 1077. https://doi.org/10.3390/ph16081077
Panova IG, Tatikolov AS. Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy). Pharmaceuticals. 2023; 16(8):1077. https://doi.org/10.3390/ph16081077
Chicago/Turabian StylePanova, Ina G., and Alexander S. Tatikolov. 2023. "Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy)" Pharmaceuticals 16, no. 8: 1077. https://doi.org/10.3390/ph16081077
APA StylePanova, I. G., & Tatikolov, A. S. (2023). Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy). Pharmaceuticals, 16(8), 1077. https://doi.org/10.3390/ph16081077