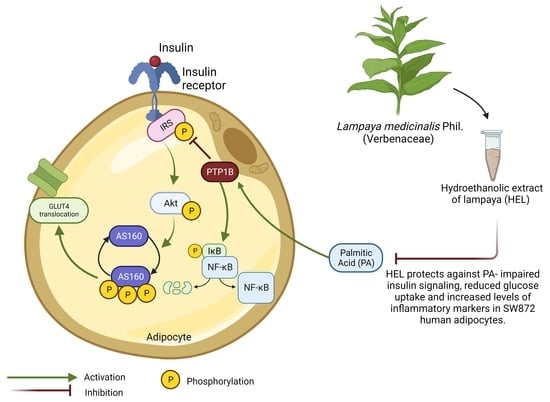
Effect of Lampaya medicinalis Phil. (Verbenaceae) and Palmitic Acid on Insulin Signaling and Inflammatory Marker Expression in Human Adipocytes
Abstract
:1. Introduction
2. Results
2.1. Adipogenic Differentiation in SW872 Cells
2.2. The Hydroethanolic Extract of Lampaya (HEL) Is Not Cytotoxic on SW872 Adipocytes
2.3. HEL Restores Impaired IRS-1 and Akt Phosphorylation Induced by PA in SW872 Adipocytes
2.4. HEL Counteracts Palmitic Acid-Impaired Glucose Uptake in SW872 Adipocytes
2.5. Effect of HEL on IκBα/NF-κB Signaling in PA-Treated SW872 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Herb Material and HEL Preparation
4.2. Culture and Differentiation of SW872 Preadipocytes
4.3. Cell Viability and Treatments
4.4. Western Blotting
4.5. Glucose Uptake
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs. Chapter 2: Towards living a longer, healthier lives. In Leaving No One Behind in an Ageing World; United Nations Publication: New York, NY, USA, 2023; pp. 35–47. [Google Scholar]
- European Commission. Horizon 2020 Work Programme 2014–2015: 8. Health, Demographic Change and Wellbeing. 2013. 2015 (10 December 2013), 95. Available online: https://ec.europa.eu/research/participants/data/ref/h2020/wp/2014_2015/main/h2020-wp1415-health_en.pdf (accessed on 27 March 2024).
- Villareal, D.T. Obesity and Accelerated Aging. J. Nutr. Health Aging 2023, 27, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Tiljak, M.K.; Reiner, Ž.; Klarica, M. Is there a better future of healthy aging? Croat. Med. J. 2020, 61, 75–78. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Ros Pérez, M.; Medina-Gómez, G. Obesity, adipogenesis and insulin resistance. Endocrinol. Y Nutr. 2011, 58, 360–369. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Bergdahl, K.; Heijbel, A.; Liljebris, C.; Bleasdale, J.E. Analysis of in vitro interactions of protein tyrosine phosphatase 1B with insulin receptors. Mol. Cell. Endocrinol. 2001, 173, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Bittner-Kowalczyk, A.; White, M.F.; Harbeck, M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 2000, 275, 4283–4289. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Darnay, B.G.; Aggarwal, B.B. Site-specific tyrosine phosphorylation of IkappaBalpha negatively regulates its inducible phosphorylation and degradation. J. Biol. Chem. 1996, 271, 31049–31054. [Google Scholar] [CrossRef]
- Benedetti, S.; Al-Tannak, N.F.; Alzharani, M.; Moir, H.J.; Stensel, D.J.; Thackray, A.E.; Naughton, D.P.; Dorak, M.T.; Spendiff, O.; Hill, N.; et al. Plasma Free Fatty Acids Metabolic Profile with LC-MS and Appetite-Related Hormones in South Asian and White European Men in Relation to Adiposity, Physical Activity and Cardiorespiratory Fitness: A Cross-Sectional Study. Metabolites 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Sabin, M.A.; De Hora, M.; Holly, J.M.; Hunt, L.P.; Ford, A.L.; Williams, S.R.; Baker, J.S.; Retallick, C.J.; Crowne, E.C.; Shield, J.P. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics 2007, 120, e1426–e1433. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Leung, J.C.; Chan, L.Y.; Yiu, W.H.; Tang, S.C. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Zhang, X.; Ke, H.; Qin, Y.; You, L.; Li, W.; Lu, G.; Chan, W.-Y.; Leung, P.C.K.; et al. Palmitic acid causes insulin resistance in granulosa cells via activation of JNK. J. Mol. Endocrinol. 2019, 62, 197–206. [Google Scholar] [CrossRef]
- Stafeev, I.S.; Michurina, S.S.; Podkuychenko, N.V.; Menshikov, M.Y.; Parfyonova, Y.V.; Vorotnikov, A.V. Chemical Inducers of Obesity-Associated Metabolic Stress Activate Inflammation and Reduce Insulin Sensitivity in 3T3-L1 Adipocytes. Biochemistry 2019, 84, 553–561. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- MohammadTaghvaei, N.; Taheripak, G.; Taghikhani, M.; Meshkani, R. Palmitate-induced PTP1B expression is mediated by ceramide-JNK and nuclear factor κB (NF-κB) activation. Cell. Signal. 2012, 24, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Suzuki, K.; Ruhee, R.T. A Brief Overview of Oxidative Stress in Adipose Tissue with a Therapeutic Approach to Taking Antioxidant Supplements. Antioxidants 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Paredes, A.; Olivares, A.; Bravo, J. Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biol. Res. 2014, 47, 6. [Google Scholar] [CrossRef]
- Morales, G.; Paredes, A. Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complement. Altern. Med. 2014, 14, 259. [Google Scholar] [CrossRef]
- Mellado, V.; Medina, E.; San Martin, C. Herbolaria Médica de Chile; Ministerio de Salud, Gobierno de Chile: Santiago, Chile, 1977; pp. 168–169. [Google Scholar]
- Sanhueza, S.; Tobar, N.; Cifuentes, M.; Quenti, D.; Varì, R.; Scazzocchio, B.; Masella, R.; Herrera, K.; Paredes, A.; Morales, G.; et al. Lampaya medicinalis Phil. decreases lipid-induced triglyceride accumulation and proinflammatory markers in human hepatocytes and fat body of Drosophila melanogaster. Int. J. Obes. 2021, 45, 1464–1475. [Google Scholar] [CrossRef]
- Ormazabal, P.; Cifuentes, M.; Varì, R.; Scazzocchio, B.; Masella, R.; Pacheco, I.; Vega, W.; Paredes, A.; Morales, G. Hydroethanolic Extract of Lampaya medicinalis Phil. (Verbenaceae) Decreases Proinflammatory Marker Expression in Palmitic Acid-exposed Macrophages. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1309–1320. [Google Scholar] [CrossRef]
- Ormazabal, P.; Herrera, K.; Cifuentes, M.; Paredes, A.; Morales, G.; Cruz, G. Protective effect of the hydroalcoholic extract from Lampaya medicinalis Phil. (Verbenaceae) on palmitic acid- impaired insulin signaling in 3T3-L1 adipocytes. Obes. Res. Clin. Pr. 2020, 14, 573–579. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Frasca, D. Several areas of overlap between obesity and aging indicate obesity as a biomarker of accelerated aging of human B cell function and antibody responses. Immun. Ageing 2022, 19, 48. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.-T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef]
- Olivieri, C.; Ruzza, M.; Tolaj, F.; DaDalt, L.; Magni, P. Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products. Biol. Life Sci. Forum 2022, 12, 19. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Lu, C.-C.; Hsu, Y.-J.; Chang, C.-J.; Lin, C.-S.; Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Lai, H.-C.; Young, J.D. Immunomodulatory properties of medicinal mushrooms: Differential effects of water and ethanol extracts on NK cell-mediated cytotoxicity. J. Endotoxin Res. 2016, 22, 522–533. [Google Scholar] [CrossRef]
- Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Chang, C.-J.; Lin, C.-S.; Lai, H.-C.; Young, J.D. Immunomodulatory Properties of Plants and Mushrooms. Trends Pharmacol. Sci. 2017, 38, 967–981. [Google Scholar] [CrossRef]
- Mfotie Njoya, E.; Eloff, J.N.; McGaw, L.J. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and antioxidant activities. BMC Complement. Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and Insulin-Resistance: From Molecular Evidences to Clinical Trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Bladé, M.C.; Salvadό, M.J.; Arola, L.; Ardévol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Shyni, G.L.; Sasidharan, K.; Francis, S.K.; Das, A.A.; Nair, M.S.; Raghu, K.G. Licarin B from Myristica fragrans improves insulin sensitivity via PPARγ and activation of GLUT4 in the IRS-1/PI3K/AKT pathway in 3T3-L1 adipocytes. RSC Adv. 2016, 6, 79859–79870. [Google Scholar] [CrossRef]
- Portman, O.W.; Alexander, M.; Maruffo, C.A. Nutritional control of arterial lipid composition in squirrel monkeys: Major ester classes and types of phospholipids. J. Nutr. 1967, 91, 35–46. [Google Scholar] [CrossRef]
- Obanda, D.N.; Hernandez, A.; Ribnicky, D.; Yu, Y.; Zhang, X.H.; Wang, Z.Q.; Cefalu, W.T. Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells. Diabetes 2012, 61, 597–605. [Google Scholar] [CrossRef]
- Ebbesson, S.O.; Tejero, M.E.; López-Alvarenga, J.C.; Harris, W.S.; Ebbesson, L.O.; Devereux, R.B.; MacCluer, J.W.; Wenger, C.; Laston, S.; Fabsitz, R.R.; et al. Individual saturated fatty acids are associated with different components of insulin resistance and glucose metabolism: The GOCADAN study. Int. J. Circumpolar Health 2010, 69, 344–351. [Google Scholar] [CrossRef]
- Obanda, D.N.; Cefalu, W.T. Modulation of cellular insulin signaling and PTP1B effects by lipid metabolites in skeletal muscle cells. J. Nutr. Biochem. 2013, 24, 1529–1537. [Google Scholar] [CrossRef]
- Muscarà, C.; Molonia, M.S.; Speciale, A.; Bashllari, R.; Cimino, F.; Occhiuto, C.; Saija, A.; Cristani, M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytotherapy Res. 2019, 33, 1888–1897. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Joubert, E.; Johnson, R.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015, 59, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Herrera, K. Efecto Protector de la Planta Lampaya medicinalis Phil. Sobre la Resistencia a la Insulina Inducida Por Ácido Palmítico en Adipocitos Diferenciados 3T3-L1; Institute of Nutrition and Food Technology (INTA), Universidad de Chile: Santiago, Chile, 2019. [Google Scholar]
- Yamamoto, N.; Ueda-Wakagi, M.; Sato, T.; Kawasaki, K.; Sawada, K.; Kawabata, K.; Akagawa, M.; Ashida, H. Measurement of Glucose Uptake in Cultured Cells. Curr. Protoc. Pharmacol. 2015, 71, 12.14.1–12.14.26. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuri, G.; Cifuentes, M.; Cisternas, P.; Paredes, A.; Ormazabal, P. Effect of Lampaya medicinalis Phil. (Verbenaceae) and Palmitic Acid on Insulin Signaling and Inflammatory Marker Expression in Human Adipocytes. Pharmaceuticals 2024, 17, 566. https://doi.org/10.3390/ph17050566
Yuri G, Cifuentes M, Cisternas P, Paredes A, Ormazabal P. Effect of Lampaya medicinalis Phil. (Verbenaceae) and Palmitic Acid on Insulin Signaling and Inflammatory Marker Expression in Human Adipocytes. Pharmaceuticals. 2024; 17(5):566. https://doi.org/10.3390/ph17050566
Chicago/Turabian StyleYuri, Gabriela, Mariana Cifuentes, Pedro Cisternas, Adrián Paredes, and Paulina Ormazabal. 2024. "Effect of Lampaya medicinalis Phil. (Verbenaceae) and Palmitic Acid on Insulin Signaling and Inflammatory Marker Expression in Human Adipocytes" Pharmaceuticals 17, no. 5: 566. https://doi.org/10.3390/ph17050566
APA StyleYuri, G., Cifuentes, M., Cisternas, P., Paredes, A., & Ormazabal, P. (2024). Effect of Lampaya medicinalis Phil. (Verbenaceae) and Palmitic Acid on Insulin Signaling and Inflammatory Marker Expression in Human Adipocytes. Pharmaceuticals, 17(5), 566. https://doi.org/10.3390/ph17050566