Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides
Abstract
:1. The Need for New Types of Contraceptives
2. Vaginally Administered Compounds with Dual Actions as Spermicides and Microbicides
2.1. Mechanisms on How Mammalian Sperm Gain Fertilizing Ability
2.2. Unsuccessful Attempts to Develop the Spermicide, Nonoxynol-9, as a Microbicide
3. Antimicrobial Peptides as Spermicides
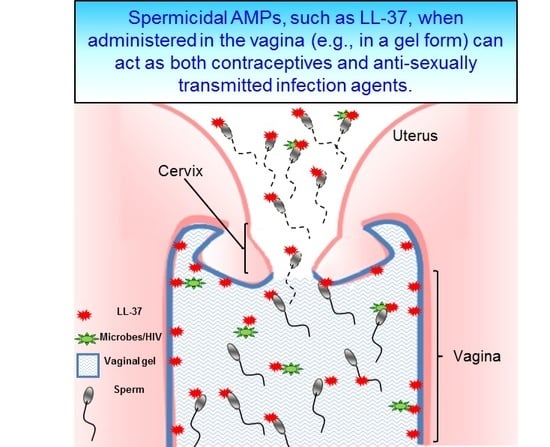
4. LL-37, the Most Promising Spermicidal AMP
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shoupe, D.; Mishell, D.R. Contraception. In Women and Health, 2nd ed.; Goldman, M.B., Troisi, R., Rexrode, K.M., Eds.; Academic Press: London, UK, 2013. [Google Scholar]
- Wadman, M. Contraceptive risk of HIV long suspected. Nature News 2011. [Google Scholar] [CrossRef]
- Spevack, E. The long-term health implications of depo-provera. Int. Med. 2013, 12, 27–34. [Google Scholar]
- Filby, A.L.; Neuparth, T.; Thorpe, K.L.; Owen, R.; Galloway, T.S.; Tyler, C.R. Health impacts of estrogens in the environment, considering complex mixture effects. Environ. Health Perspect. 2007, 115, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.; O’Brien, K.; Woodruff, T. Are oral contraceptives a significant contributor to the estrogenicity of drinking water? Environ. Sci. Technol. 2011, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.T. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 2011, 62, 127–139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Annual technical report. Department of Reproductive Health and Research, 2013. [Google Scholar]
- Florman, H.; Fissore, R. Fertilization in mammals. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A., Eds.; Elsevier Inc.: New York, NY, USA, 2015; pp. 149–195. [Google Scholar]
- Travis, A.J.; Kopf, G.S. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Investig. 2002, 110, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M. The cell biology of mammalian fertilization. Development. 2013, 140, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update. 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.J.; Clapham, D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Navarro, B.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. A novel gene required for male fertility and functional Catsper channel formation in spermatozoa. Nature Commun. 2011, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Carmona, E.; Bou Khalil, M.; Xu, H.; Berger, T.; Gerton, G.L. New insights into sperm-zona pellucida interaction: Involvement of sperm lipid rafts. Front. Biosci. 2007, 12, 1748–1766. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Weerachatyanukul, W.; Gadella, B.; Kamolvarin, N.; Attar, M.; Tanphaichitr, N. Role of sperm sulfogalactosylglycerolipid in mouse sperm-zona pellucida binding. Biol. Reprod. 2000, 63, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Weerachatyanukul, W.; Rattanachaiyanont, M.; Carmona, E.; Furimsky, A.; Mai, A.; Shoushtarian, A.; Sirichotiyakul, S.; Ballakier, H.; Leader, A.; Tanphaichitr, N. Sulfogalactosylglycerolipid is involved in human gamete interaction. Mol. Reprod. Dev. 2001, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Inoue, N.; Benham, A.M.; Okabe, M. Fertilization: A sperm’s journey to and interaction with the oocyte. J. Clin. Investig. 2010, 120, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Lyng, R.; Shur, B.D. Sperm-egg binding requires a multiplicity of receptor-ligand interactions: New insights into the nature of gamete receptors derived from reproductive tract secretions. Soc. Reprod. Fertil. Suppl. 2007, 65, 335–351. [Google Scholar] [PubMed]
- Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J.; Whitelegge, J.; et al. Proteomic characterization of pig sperm anterior head plasma membrane reveals roles of acrosomal proteins in ZP3 binding. J. Cell Physiol. 2015, 230, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J.; et al. Remodeling of the plasma membrane in preparation for sperm-egg recognition: Roles of acrosomal proteins. Asian J. Androl. 2015, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Munch, J.; Rucker, E.; Standker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Chijioke, P.C.; Zaman, S.; Pearson, R.M. Comparison of the potency of d-propanolol, chlorhexidine and nonoxynol-9 in the sander cramer test. Contraception 1986, 34, 207–211. [Google Scholar] [CrossRef]
- Dunmire, E.N.; Katz, D.F. Kinematic response of human spermatozoa to nonoxynol-9. Biol. Reprod. 1994, 50, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Malamud, D.; Storey, B.T. Assessment of the anti-microbial agent c31g as a spermicide: Comparison with nonoxynol-9. Contraception 1996, 53, 313–318. [Google Scholar] [CrossRef]
- Asculai, S.S.; Weis, M.T.; Rancourt, M.W.; Kupferberg, A.B. Inactivation of herpes simplex viruses by nonionic surfactants. Antimicrob. Agents Chemother. 1978, 13, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Whaley, K.J.; Mandrell, T.D.; Bishop, B.D.; Witt, C.J.; Cone, R.A. The cat/feline immunodeficiency virus model for transmucosal transmission of aids: Nonoxynol-9 contraceptive jelly blocks transmission by an infected cell inoculum. AIDS 1993, 7, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Benes, S.; McCormack, W.M. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vivo. Antimicrob. Agents Chemother. 1985, 27, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Malkovsky, M.; Newell, A.; Dalgleish, A.G. Inactivation of HIV by nonoxynol-9. Lancet 1988, 1, 645. [Google Scholar] [CrossRef]
- Doncel, G.F. Exploiting common targets in human fertilization and HIV infection: Development of novel contraceptive microbicides. Hum. Reprod. Update 2006, 12, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Alexander, N.J.; Gettie, A.; Hendrickx, A.G.; Marx, P.A. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in Rhesus macaques. Fertil. Steril. 1992, 57, 1126–1128. [Google Scholar] [PubMed]
- Weber, J.; Nunn, A.; O’Connor, T.; Jeffries, D.; Kitchen, V.; McCormack, S.; Stott, J.; Almond, N.; Stone, A.; Darbyshire, J. Chemical condoms’ for the prevention of HIV infection: Evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS 2001, 15, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Tholandi, M.; Ramjee, G.; Rutherford, G.W. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: Systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect. Dis. 2002, 2, 613–617. [Google Scholar] [CrossRef]
- Van Damme, L.; Ramjee, G.; Alary, M.; Vuylsteke, B.; Chandeying, V.; Rees, H.; Sirivongrangson, P.; Mukenge-Tshibaka, L.; Ettiegne-Traore, V.; Uaheowitchai, C.; et al. Effectiveness of col-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet 2002, 360, 971–977. [Google Scholar] [CrossRef]
- Richardson, B.A.; Lavreys, L.; Martin, H.L., Jr.; Stevens, C.E.; Ngugi, E.; Mandaliya, K.; Bwayo, J.; Ndinya-Achola, J.; Kreiss, J.K. Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: A randomized clinical trial. Sex. Transm. Dis. 2001, 28, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Stafford, M.K.; Ward, H.; Flanagan, A.; Rosenstein, I.J.; Taylor-Robinson, D.; Smith, J.R.; Weber, J.; Kitchen, V.S. Safety study of nonoxynol-9 as a vaginal microbicide: Evidence of adverse effects. J. Acquir. Immune Defic. Syndr. 1998, 17, 327–331. [Google Scholar] [CrossRef]
- Schreiber, C.A.; Meyn, L.A.; Creinin, M.D.; Barnhart, K.T.; Hillier, S.L. Effects of long-term use of nonoxynol-9 on vaginal flora. Obstet. Gynecol. 2006, 107, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Effects of the spermicidal agent nonoxynol-9 on vaginal microbial flora. J. Infect. Dis. 1992, 165, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L., Jr.; Stevens, C.E.; Richardson, B.A.; Rugamba, D.; Nyange, P.M.; Mandaliya, K.; Ndinya-Achola, J.; Kreiss, J.K. Safety of a nonoxynol-9 vaginal gel in Kenyan prostitutes. A randomized clinical trial. Sex. Transm. Dis. 1997, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, J.; Ngugi, E.; Holmes, K.; Ndinya-Achola, J.; Waiyaki, P.; Roberts, P.L.; Ruminjo, I.; Sajabi, R.; Kimata, J.; Fleming, T.R.; et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 1992, 268, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Desai, K.; Darbyshire, J.; Microbicides Development Program. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med 2005, 2, e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niruthisard, S.; Roddy, R.E.; Chutivongse, S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 1991, 18, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Lozenski, K.; Ownbey, R.; Wigdahl, B.; Kish-Catalone, T.; Krebs, F.C. Decreased cervical epithelial sensitivity to nonoxynol-9 (N-9) after four daily applications in a murine model of topical vaginal microbicide safety. BMC Pharmacol. Toxicol. 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Tucker, L.D.; Anderson, D.J. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 2001, 184, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Reardon, S. Bacterial arms race revs up. Nature 2015, 521, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Nijnik, A.; Hancock, R.E.W. Host defense peptides: Bridging antimicrobial and immunomodulatory activities. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Lui, H.W., Eds.; Elsevier Science: Oxford, UK, 2010; Volume 5, pp. 175–216. [Google Scholar]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, H.K.; Lee, H.R.; Chung, C.P.; Choi, B.K. Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int. J. Antimicrob Agents 2010, 35, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [PubMed]
- Molhoek, E.M.; den Hertog, A.L.; de Vries, A.M.; Nazmi, K.; Veerman, E.C.; Hartgers, F.C.; Yazdanbakhsh, M.; Bikker, F.J.; van der Kleij, D. Structure-function relationship of the human antimicrobial peptide LL-37 and LL-37 fragments in the modulation of TLR responses. Biol. Chem. 2009, 390, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.; Davidson, D.J.; Lau, Y.E.; Lee, K.; Scott, M.G.; Hancock, R.E. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 2005, 77, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Mookherjee, N. Multiple immune-modulatory functions of cathelicidin host defense peptides. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Kraneburg, U.; Jacobsen, F.; Al Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 2011, 216, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Escamez, M.J.; Garcia, M.; Duarte, B.; Holguin, A.; Retamosa, L.; Jorcano, J.L.; Rio, M.D.; Larcher, F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Investig. Dermatol. 2008, 128, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Tokumaru, S.; Sayama, K.; Shirakata, Y.; Komatsuzawa, H.; Ouhara, K.; Hanakawa, Y.; Yahata, Y.; Dai, X.; Tohyama, M.; Nagai, H.; et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005, 175, 4662–4668. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Hirsch, T.; Schulte, M.; Kueckelhaus, M.; Jacobsen, F.; Mersch, E.A.; Stricker, I.; Afacan, N.; Jenssen, H.; Hancock, R.E.; et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE 2012, 7, e39373. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krotz, F.; Zahler, S.; Gloe, T.; Issbrucker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Lau, K.; Lushnikova, T.; Golla, R.; Wang, X. Antimicrobial peptides in 2014. Pharmaceuticals 2015, 8, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zasloff, M. Part 1: Natural antimicrobial peptides: Nomenclature, classification and interesting templates for peptide engineering. In Antimicrobial peptides, Discovery, Design and Novel Therapeutic Strategies, 1st ed.; Wang, G., Ed.; CABI: Oxfordshire, UK, 2010; pp. 1–21. [Google Scholar]
- Wang, G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol. Biol. 2015, 1268, 43–66. [Google Scholar] [PubMed]
- Chatterjee, C.; Paul, M.; Xie, L.; van der Donk, W.A. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 2005, 105, 633–684. [Google Scholar] [CrossRef] [PubMed]
- Ketchem, R.R.; Lee, K.C.; Huo, S.; Cross, T.A. Macromolecular structural elucidation with solid-state NMR-derived orientational constraints. J. Biomol. NMR 1996, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lohans, C.T.; Li, J.L.; Vederas, J.C. Structure and biosynthesis of carnolysin, a homologue of enterococcal cytolysin with d-amino acids. J. Am. Chem. Soc. 2014, 136, 13150–13153. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta 2014, 1838, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Noda, Y.D.; Fujimoto, M.; Nicolson, G.L. The distribution of negative surface charges on mammalian spermatozoa. Am. J. Anat. 1972, 135, 497–519. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.; Nixon, B.; Aitken, R.J. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum. Reprod. 2005, 20, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Bou Khalil, M.; Weerachatyanukul, W.; Kates, M.; Xu, H.; Carmona, E.; Attar, M.; Carrier, D. Physiological and biophysical properties of male germ cell sulfogalactosylglycerolipid. In Lipid Metabolism and Male Fertility; De Vriese, S., Ed.; AOCS Press: Champaign, IL, USA, 2003; Volume 11, pp. 125–148. [Google Scholar]
- Kongmanas, K.; Xu, H.; Yaghoubian, A.; Franchini, L.; Panza, L.; Ronchetti, F.; Faull, K.; Tanphaichitr, N. Quantification of seminolipid by LC-ESI-MS/MS-multiple reaction monitoring: Compensatory levels in Cgt(+/−) mice. J. Lipid Res. 2010, 51, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- Bou Khalil, M.; Chakrabandhu, K.; Xu, H.; Weerachatyanukul, W.; Buhr, M.; Berger, T.; Carmona, E.; Vuong, N.; Kumarathasan, P.; Wong, P.T.; et al. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev. Biol. 2006, 290, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Attar, M.; Kates, M.; Bou Khalil, M.; Carrier, D.; Wong, P.T.T.; Tanphaichitr, N. A fourier-transform infrared study of the interaction between germ-cell specific sulfogalactosylglycerolipid and dimyristoylglycerophosphocholine. Chem. Phys. Lipids 2000, 106, 101–114. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Faull, K.F.; Yaghoubian, A.; Xu, H. Lipid rafts and sulfogalactosylglycerolipid (SGG) in sperm functions: Consensus and controversy. Trends Glycosci. Glycotech. 2007, 19, 67–83. [Google Scholar] [CrossRef]
- Simon, P.; Baumner, S.; Busch, O.; Rohrich, R.; Kaese, M.; Richterich, P.; Wehrend, A.; Muller, K.; Gerardy-Schahn, R.; Muhlenhoff, M.; et al. Polysialic acid is present in mammalian semen as a post-translational modification of the neural cell adhesion molecule NCAM and the polysialyltransferase ST8Siall. J. Biol. Chem. 2013, 288, 18825–18833. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Schroter, S. New insights into the origin, structure and role of CD52: A major component of the mammalian sperm glycocalyx. Cells Tiss. Organs 2001, 168, 93–104. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, F.; Verardi, R.; Shi, L.; Henzler-Wildman, K.A.; Ramamoorthy, A.; Veglia, G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry 2008, 47, 5565–5572. [Google Scholar] [CrossRef] [PubMed]
- Srakaew, N.; Young, C.D.; Sae-wu, A.; Xu, H.; Quesnel, K.L.; di Brisco, R.; Kongmanas, K.; Fongmoon, D.; Hommalai, G.; Weerachatyanukul, W.; et al. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum. Reprod. 2014, 29, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Zheng, Y.T.; Shen, J.H.; Liu, G.J.; Liu, H.; Lee, W.H.; Tang, S.Z.; Zhang, Y. Antimicrobial peptides from skin secretions of chinese red belly toad Bombina maxima. Peptides 2002, 23, 427–435. [Google Scholar] [CrossRef]
- Toke, O.; Banoczi, Z.; Kiraly, P.; Heinzmann, R.; Burck, J.; Ulrich, A.S.; Hudecz, F. A kinked antimicrobial peptide from Bombina maxima. I. Three-dimensional structure determined by NMR in membrane-mimicking environments. Eur. Biophys. J. 2011, 40, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Clara, A.; Manjramkar, D.D.; Reddy, V.K. Preclinical evaluation of magainin-a as a contraceptive antimicrobial agent. Fertil. Steril. 2004, 81, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Gesell, J.; Zasloff, M.; Opella, S.J. Two-dimensional 1h nmr experiments show that the 23-residue magainin antibiotic peptide is an alpha-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. J. Biomol. NMR 1997, 9, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.R.; Manjramkar, D.D. Evaluation of the antifertility effect of magainin-a in rabbits: In vitro and in vivo studies. Fertil. Steril. 2000, 73, 353–358. [Google Scholar] [CrossRef]
- Edelstein, M.C.; Gretz, J.E.; Bauer, T.J.; Fulgham, D.L.; Alexander, N.J.; Archer, D.F. Studies on the in vivo spermicidal activity of synthetic magainins. Fertil. Steril. 1991, 55, 647–649. [Google Scholar] [PubMed]
- Reddy, K.V.; Shahani, S.K.; Meherji, P.K. Spermicidal activity of magainins: In vitro and in vivo studies. Contraception 1996, 53, 205–210. [Google Scholar] [CrossRef]
- Wojcik, C.; Sawicki, W.; Marianowski, P.; Benchaib, M.; Czyba, J.C.; Guerin, J.F. Cyclodextrin enhances spermicidal effects of magainin-2-amide. Contraception 2000, 62, 99–103. [Google Scholar] [CrossRef]
- Chen, H.C.; Brown, J.H.; Morell, J.L.; Huang, C.M. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 1988, 236, 462–466. [Google Scholar] [CrossRef]
- Mor, A.; Nicolas, P. The NH2-terminal alpha-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 1994, 269, 1934–1939. [Google Scholar] [PubMed]
- Zairi, A.; Belaid, A.; Gahbiche, A.; Hani, K. Spermicidal activity of dermaseptins. Contraception 2005, 72, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Shalev, D.E.; Rotem, S.; Fish, A.; Mor, A. Consequences of N-acylation on structure and membrane binding properties of dermaseptin derivative K4-S4-(1-13). J. Biol Chem. 2006, 281, 9432–9438. [Google Scholar] [CrossRef] [PubMed]
- Zairi, A.; Serres, C.; Tangy, F.; Jouannet, P.; Hani, K. In vivo spermicidal activity of peptides from amphibian skin: Dermaseptin S4 and derivatives. Bioorg. Med. Chem. 2008, 16, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zairi, A.; Tangy, F.; Bouassida, K.; Hani, K. Dermaseptins and magainins: Antimicrobial peptides from frogs’ skin-new sources for a promising spermicides microbicides-a mini review. J. Biomed. Biotechnol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Fesahat, F.; Anbari, F.; Halvaei, I.; Ebrahimi, L. Assessment of spermicidal activity of the antimicrobial peptide sarcotoxin Pd: A potent contraceptive agent. Eur. J. Contracept. Reprod. Health Care 2015, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hasper, H.E.; Kramer, N.E.; Smith, J.L.; Hillman, J.D.; Zachariah, C.; Kuipers, O.P.; de Kruijff, B.; Breukink, E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 2006, 313, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hooven, H.W.; Doeland, C.C.; Van De Kamp, M.; Konings, R.N.; Hilbers, C.W.; Van De Ven, F.J. Three-dimensional structure of the lantibiotic nisin in the presence of membrane-mimetic micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur. J. Biochem. 1996, 235, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hooven, H.W.; Spronk, C.A.; Van De Kamp, M.; Konings, R.N.; Hilbers, C.W.; Van De Van, F.J. Surface location and orientation of the lantibiotic nisin bound to membrane-mimicking micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur. J. Biochem. 1996, 235, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Aranha, C.; Gupta, S.; Reddy, K.V. Contraceptive efficacy of antimicrobial peptide Nisin: In vitro and in vivo studies. Contraception 2004, 69, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Aranha, C.; Gupta, S.M.; Yedery, R.D. Evaluation of antimicrobial peptide nisin as a safe vaginal contraceptive agent in rabbits: In vitro and in vivo studies. Reproduction 2004, 128, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Aranha, C.C.; Gupta, S.M.; Reddy, K.V. Assessment of cervicovaginal cytokine levels following exposure to microbicide nisin gel in rabbits. Cytokine 2008, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Silkin, L.; Hamza, S.; Kaufman, S.; Cobb, S.L.; Vederas, J.C. Spermicidal bacteriocins: Lacticin 3147 and subtilosin a. Bioorg. Med. Chem. Lett. 2008, 18, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Aranha, C.C.; Bellare, J.R.; Reddy, K.V. Interaction of contraceptive antimicrobial peptide nisin with target cell membranes: Implications for use as vaginal microbicide. Contraception 2009, 80, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Gupta, S.M.; Aranha, C.C. Effect of antimicrobial peptide, nisin, on the reproductive functions of rats. ISRN Vet. Sci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Balgir, P.P.; Kaur, B.; Mittu, B.; Garg, N. Antimicrobial and spermicidal activity of native and recombinant pediocin CP2: A camparative evaluation. Arch. Clin. Microbiol. 2012, 3, 1–12. [Google Scholar]
- Bourinbaiar, A.S.; Krasinski, K.; Borkowsky, W. Anti-HIV effect of gramicidin in vivo: Potential for spermicide use. Life Sci. 1994, 54, 5–9. [Google Scholar] [CrossRef]
- Lee, C.H.; Bagdon, R.; Chien, Y.W. Comparative in vivo spermicidal activity of chelating agents and synergistic effect with nonoxynol-9 on human sperm functionality. J. Pharm. Sci. 1996, 85, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Centola, G.M. Dose-response effects of gramicidin-D, EDTA, and nonoxynol-9 on sperm motion parameters and acrosome status. Contraception 1998, 58, 35–38. [Google Scholar] [CrossRef]
- Kawulka, K.E.; Sprules, T.; Diaper, C.M.; Whittal, R.M.; McKay, R.T.; Mercier, P.; Zuber, P.; Vederas, J.C. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to alpha-carbon cross-links: Formation and reduction of alpha-thio-alpha-amino acid derivatives. Biochemistry 2004, 43, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Sutyak, K.E.; Anderson, R.A.; Dover, S.E.; Feathergill, K.A.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect. Dis. Obstet. Gynecol. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.I.; Sprules, T.; Carpenter, M.R.; Cotter, P.D.; Hill, C.; Ross, R.P.; Vederas, J.C. Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 2004, 43, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Ballweber, L.M.; Jaynes, J.E.; Stamm, W.E.; Lampe, M.F. In vivo microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 2002, 46, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Randall, M.; Fitzgerald, L.; Lee, G.; Seibel, M.; Taymor, M. An increase in in vivo fertilization ability of low-density human sperm capacitated by multiple-tube swim up. Fert. Steril. 1987, 48, 821–827. [Google Scholar]
- Tanphaichitr, N.; Millette, C.F.; Agulnick, A.; Fitzgerald, L.M. Egg-penetration ability and structural properties of human sperm prepared by percoll-gradient centrifugation. Gamete Res. 1988, 20, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.F.; Drobnis, E.Z.; Overstreet, J.W. Factors regulating mammalian sperm migration through the female reproductive tract and oocyte vestments. Gamete Res. 1989, 22, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Gamete and zygote transport. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A., Eds.; Elsevier Inc.: New York, NY, USA, 2015; pp. 113–145. [Google Scholar]
- Noguchi, K.; Tsukumi, K.; Urano, T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp. Med. 2003, 53, 404–412. [Google Scholar] [PubMed]
- Ronnqvist, P.D.; Forsgren-Brusk, U.B.; Grahn-Hakansson, E.E. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet. Gynecol. Scand. 2006, 85, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.; Olson, M.E.; Crichlow, A.M.; Osborne, A.D.; Costerton, J.W. The normal microflora of the female rabbit’s genital tract. Can. J. Vet. Res. 1986, 50, 272–274. [Google Scholar] [PubMed]
- Cohen, L. Influence of pH on vaginal discharges. Br. J. Vener. Dis. 1969, 45, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tevi-Benissan, C.; Belec, L.; Levy, M.; Schneider-Fauveau, V.; Si Mohamed, A.; Hallouin, M.C.; Matta, M.; Gresenguet, G. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 1997, 4, 367–374. [Google Scholar] [PubMed]
- Castle, P.E.; Hoen, T.E.; Whaley, K.J.; Cone, R.A. Contraceptive testing of vaginal agents in rabbits. Contraception 1998, 58, 51–60. [Google Scholar] [CrossRef]
- Meysick, K.C.; Garber, G.E. Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J. Parasitol. 1992, 78, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Rheinwald, J.G.; Anderson, D.J. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 1997, 57, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ayehunie, S.; Cannon, C.; Lamore, S.; Kubilus, J.; Anderson, D.J.; Pudney, J.; Klausner, M. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol. In Vitro 2006, 20, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zalenskaya, I.A.; Joseph, T.; Bavarva, J.; Yousefieh, N.; Jackson, S.S.; Fashemi, T.; Yamamoto, H.S.; Settlage, R.; Fichorova, R.N.; Doncel, G.F. Gene expression profiling of human vaginal cells in vivo discriminates compounds with pro-inflammatory and mucosa-altering properties: Novel biomarkers for preclinical testing of HIV microbicide candidates. PLoS ONE 2015, 10, e0128557. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C.; Miller, S.R.; Catalone, B.J.; Fichorova, R.; Anderson, D.; Malamud, D.; Howett, M.K.; Wigdahl, B. Comparative in vivo sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob. Agents Chemother. 2002, 46, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Catalone, B.J.; Kish-Catalone, T.M.; Budgeon, L.R.; Neely, E.B.; Ferguson, M.; Krebs, F.C.; Howett, M.K.; Labib, M.; Rando, R.; Wigdahl, B. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 2004, 48, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Bon, I.; Lembo, D.; Rusnati, M.; Clo, A.; Morini, S.; Miserocchi, A.; Bugatti, A.; Grigolon, S.; Musumeci, G.; Landolfo, S.; et al. Peptide-derivatized SB105-A10 dendrimer inhibits the infectivity of R5 and X4 HIV-1 strains in primary pbmcs and cervicovaginal histocultures. PLoS ONE 2013, 8, e76482. [Google Scholar] [CrossRef] [PubMed]
- Lagenaur, L.A.; Sanders-Beer, B.E.; Brichacek, B.; Pal, R.; Liu, X.; Liu, Y.; Yu, R.; Venzon, D.; Lee, P.P.; Hamer, D.H. Prevention of vaginal SHIV transmission in macaques by a live recombinant lactobacillus. Mucosal Immunol. 2011, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Simmons, A.P.; Ugaonkar, S.R.; Watson, K.M.; Dezzutti, C.S.; Rohan, L.C.; Buckheit, R.W., Jr.; Kiser, P.F. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob. Agents Chemother. 2011, 55, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.R. Hela cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural antimicrobials and their role in vaginal health: A short review. Int. J. Probiotics Prebiotics 2008, 3, 219–230. [Google Scholar] [PubMed]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Soderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef] [PubMed]
- Levinson, P.; Choi, R.Y.; Cole, A.L.; Hirbod, T.; Rhedin, S.; Payne, B.; Guthrie, B.L.; Bosire, R.; Cole, A.M.; Farquhar, C.; et al. HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS ONE 2012, 7, e31996. [Google Scholar] [CrossRef] [PubMed]
- Lorin, C.; Saidi, H.; Belaid, A.; Zairi, A.; Baleux, F.; Hocini, H.; Belec, L.; Hani, K.; Tangy, F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vivo. Virology 2005, 334, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.T.; Breukink, E.; Tischenko, E.; Lutters, M.A.; de Kruijff, B.; Kaptein, R.; Bonvin, A.M.; van Nuland, N.A. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 2004, 11, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution structures of human LL-37 fragments and nmr-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.F.; Wang, G.; Berno, B.; Epand, R.M. Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob. Agents Chemother. 2009, 53, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Breukink, E. Antimicrobial peptides produced by microorganisms. In Antimicrobial Peptides and Innate Immunity; Hiemstra, P.S., Zaat, S.A.J., Eds.; Springer: Basel, Switzerland, 2013; pp. 53–95. [Google Scholar]
- Wang, G.; Li, X.; Wang, Z. Apd3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Koradi, R.; Billeter, M.; Wuthrich, K. Molmol: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Durr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Zelezetsky, I.; Pontillo, A.; Puzzi, L.; Antcheva, N.; Segat, L.; Pacor, S.; Crovella, S.; Tossi, A. Evolution of the primate cathelicidin. Correlation between structural variations and antimicrobial activity. J. Biol. Chem. 2006, 281, 19861–19871. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Johnsen, A.H.; Borregaard, N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995, 368, 173–176. [Google Scholar] [CrossRef]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jornvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [PubMed]
- Di Nardo, A.; Vitiello, A.; Gallo, R.L. Cutting edge: Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003, 170, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Frohm Nilsson, M.; Sandstedt, B.; Sorensen, O.; Weber, G.; Borregaard, N.; Stahle-Backdahl, M. The human cationic antimicrobial protein (hCAP-18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 1999, 67, 2561–2566. [Google Scholar] [PubMed]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Sorensen, O.; Persson, T.; Frohm-Nilsson, M.; Johansson, B.; Bjartell, A.; Lilja, H.; Stahle-Backdahl, M.; Borregaard, N.; Egesten, A. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 2000, 68, 4297–4302. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsboll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003, 278, 28540–28546. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Slamova, Z.; Bergman, P.; Kovacs, L.; Podracka, L.; Ehren, I.; Hokfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.L.; Dynesen, P.; Larsen, P.; Jakobsen, L.; Andersen, P.S.; Frimodt-Moller, N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect. Immun. 2014, 82, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Eckmann, L.; Leopard, J.D.; Varki, N.; Kagnoff, M.F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 2002, 70, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.S.; Rabe, K.F.; Hiemstra, P.S. The human cathelicidin LL-37: A multifunctional peptide involved in infection and inflammation in the lung. Pulm. Pharmacol. Ther. 2005, 18, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.; Zenobia, C.; Darveau, R.P. Defensins and LL-37: A review of function in the gingival epithelium. Periodontology 2000 2013, 63, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, A.; Olliver, M.; Rekha, R.S.; Lindh, M.; Lindbom, L.; Normark, S.; Henriques-Normark, B.; Andersson, J.; Agerberth, B.; Bergman, P. Impaired release of antimicrobial peptides into nasal fluid of hyper-IgE and CVID patients. PLoS ONE 2011, 6, e29316. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.J.; Tjabringa, G.S.; Vonk, M.J.; Hiemstra, P.S.; Grote, J.J. Bacterial products increase expression of the human cathelicidin hCap-18/LL-37 in cultured human sinus epithelial cells. FEMS Immunol Med. Microbiol. 2004, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human defensins and LL-37 in mucosal immunity. J. Leukoc. Biol. 2010, 87, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Reinherz, E.L. Dynamic recruitment of human CD2 into lipid rafts. Linkage to T cell signal transduction. J. Biol. Chem. 2001, 276, 18775–18785. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Sorensen, O.E.; Frohm, B.; Borregaard, N.; Egesten, A.; Malm, J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum Reprod. 2002, 17, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Cottell, E.; Harrison, R.F.; McCaffrey, M.; Walsh, T.; Mallon, E.; Barry-Kinsella, C. Are seminal fluid microorganisms of significance or merely contaminants? Fertil. Steril. 2000, 74, 465–470. [Google Scholar] [CrossRef]
- Smeianov, V.; Scott, K.; Reid, G. Activity of cecropin p1 and FA-LL-37 against urogenital microflora. Microbes Infect. 2000, 2, 773–777. [Google Scholar] [CrossRef]
- Sambri, V.; Marangoni, A.; Giacani, L.; Gennaro, R.; Murgia, R.; Cevenini, R.; Cinco, M. Comparative in vivo activity of five cathelicidin-derived synthetic peptides against leptospira, borrelia and Treponema pallidum. J. Antimicrob. Chemother. 2002, 50, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Takagi, S.; Guo, Y.; Kuroda, K.; Ando, T.; Yoneyama, H.; Ito, K.; Isogai, E. Inhibition of Streptococcus mutans biofilm by LL-37. Int. J. Med. Sci. Biotechnol. 2013, 1, 56–64. [Google Scholar]
- Moffatt, J.H.; Harper, M.; Mansell, A.; Crane, B.; Fitzsimons, T.C.; Nation, R.L.; Li, J.; Adler, B.; Boyce, J.D. Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect. Immun. 2013, 81, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintanilla, M.; Pulido, M.R.; Moreno-Martinez, P.; Martin-Pena, R.; Lopez-Rojas, R.; Pachon, J.; McConnell, M.J. Activity of host antimicrobials against multidrug-resistant Acinetobacter baumannii acquiring colistin resistance through loss of lipopolysaccharide. Antimicrob. Agents Chemother. 2014, 58, 2972–2975. [Google Scholar] [CrossRef] [PubMed]
- Ouhara, K.; Komatsuzawa, H.; Yamada, S.; Shiba, H.; Fujiwara, T.; Ohara, M.; Sayama, K.; Hashimoto, K.; Kurihara, H.; Sugai, M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, beta-defensins and LL-37, produced by human epithelial cells. J. Antimicrob. Chemother. 2005, 55, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Miyasaki, K.T.; Lehrer, R.I. Sensitivity of actinobacillus actinomycetemcomitans and Capnocytophaga spp. To the bactericidal action of LL-37: A cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 2000, 15, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Thwaite, J.E.; Hibbs, S.; Titball, R.W.; Atkins, T.P. Proteolytic degradation of human antimicrobial peptide LL-37 by Bacillus anthracis may contribute to virulence. Antimicrob. Agents Chemother. 2006, 50, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Lusitani, D.; Malawista, S.E.; Montgomery, R.R. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 2002, 185, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kanthawong, S.; Nazmi, K.; Wongratanacheewin, S.; Bolscher, J.G.; Wuthiekanun, V.; Taweechaisupapong, S. In vitro susceptibility of Burkholderia pseudomallei to antimicrobial peptides. Int. J. Antimicrob. Agents 2009, 34, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kanthawong, S.; Bolscher, J.G.; Veerman, E.C.; van Marle, J.; Nazmi, K.; Wongratanacheewin, S.; Taweechaisupapong, S. Antimicrobial activities of LL-37 and its truncated variants against Burkholderia thailandensis. Int. J. Antimicrob. Agents 2010, 36, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Cheng, Y.L.; Hsieh, W.P.; Lan, C.Y. Responses of Candida albicans to the human antimicrobial peptide LL-37. J. Microbiol. 2014, 52, 581–589. [Google Scholar] [CrossRef]
- Tang, L.; Chen, J.; Zhou, Z.; Yu, P.; Yang, Z.; Zhong, G. Chlamydia-secreted protease CPAF degrades host antimicrobial peptides. Microbes Infect. 2015, 17, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.; Roxas, B.; Viswanathan, V.K.; Vedantam, G. Clostridium difficile clinical isolates exhibit variable susceptibility and proteome alterations upon exposure to mammalian cationic antimicrobial peptides. Anaerobe 2012, 18, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, K.; Namiot, D.; Byfield, F.J.; Cruz, K.; Zendzian-Piotrowska, M.; Fein, D.E.; Savage, P.B.; Diamond, S.; McCulloch, C.A.; Janmey, P.A.; et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J. Antimicrob. Chemother. 2013, 68, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Mattiuzzo, M.; Herasimenka, Y.; Cescutti, P.; Rizzo, R.; Gennaro, R. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J. Peptide Sci. 2009, 15, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, K.; Namiot, A.; Fein, D.E.; Wen, Q.; Namiot, Z.; Savage, P.B.; Diamond, S.; Janmey, P.A.; Bucki, R. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kai-Larsen, Y.; Luthje, P.; Chromek, M.; Peters, V.; Wang, X.; Holm, A.; Kadas, L.; Hedlund, K.O.; Johansson, J.; Chapman, M.R.; et al. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 2010, 6, e1001010. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Kuwahara-Arai, K.; Tamura, H.; Hiramatsu, K.; Hirata, M. Augmentation of the bactericidal activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by amino acid substitutions. Inflamm. Res. 2005, 54, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, E.S.; Gould, J.; Bals, R.; Wilson, J.M.; Weiser, J.N. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 2000, 68, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Wang, G.; White, M.; Qi, L.; Taubenberger, J.; Hartshorn, K.L. Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic influenza a viruses. PLoS One 2015, 10, e0124706. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- De Majumdar, S.; Yu, J.; Fookes, M.; McAteer, S.P.; Llobet, E.; Finn, S.; Spence, S.; Monahan, A.; Kissenpfennig, A.; Ingram, R.J.; et al. Elucidation of the rama regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog. 2015, 11, e1004627. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Johansson, L.; Asp, V.; Plant, L.; Gudmundsson, G.H.; Jonsson, A.B.; Agerberth, B. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell. Microbiol. 2005, 7, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Georg, M.; Maudsdotter, L.; Jonsson, A.B. Endotoxin, capsule, and bacterial ttachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 2009, 191, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Reeves, E.P.; McNally, P.; Chotirmall, S.H.; Greene, C.M.; Greally, P.; Murphy, P.; O’Neill, S.J.; McElvaney, N.G. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 2009, 183, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Susceptibility of Pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Dosler, S.; Karaaslan, E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.M.; Findlay, E.G.; McHugh, B.J.; Mackellar, A.; Man, T.; Macmillan, D.; Wang, H.; Fitch, P.M.; Schwarze, J.; Davidson, D.J. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS ONE 2013, 8, e73659. [Google Scholar] [CrossRef] [PubMed]
- Noore, J.; Noore, A.; Li, B. Cationic antimicrobial peptide LL-37 is effective against both extra- and intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Senyurek, I.; Paulmann, M.; Sinnberg, T.; Kalbacher, H.; Deeg, M.; Gutsmann, T.; Hermes, M.; Kohler, T.; Gotz, F.; Wolz, C.; et al. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Crabb, D.M.; Dai, Y.; Chen, Y.; Waites, K.B.; Atkinson, T.P. Suppression of antimicrobial peptide expression by ureaplasma species. Infect. Immun. 2014, 82, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Jones, J.F.; Kisich, K.O.; Streib, J.E.; Gallo, R.L.; Leung, D.Y. Selective killing of vaccinia virus by LL-37: Implications for eczema vaccinatum. J. Immunol. 2004, 172, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Crack, L.R.; Jones, L.; Malavige, G.N.; Patel, V.; Ogg, G.S. Human antimicrobial peptides LL-37 and human beta-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 2012, 37, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Ross, T.; Pitout, J.D.; Church, D.L.; Gregson, D.B. Community-onset urinary tract infections: A population-based assessment. Infection 2007, 35, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M.; Polyak, F.; Postel, E. Periurethral bacterial flora in women. Prolonged intermittent colonization with Escherichia coli. JAMA 1980, 243, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Langlais, J.; Kan, F.W.; Granger, L.; Raymond, L.; Bleau, G.; Roberts, K.D. Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation. Gamete Res. 1988, 20, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Hamil, K.G.; Sivashanmugam, P.; Richardson, R.T.; Grossman, G.; Ruben, S.M.; Mohler, J.L.; Petrusz, P.; O’Rand, M.G.; French, F.S.; Hall, S.H. HE2beta and HE2gamma, new members of an epididymis-specific family of androgen-regulated proteins in the human. Endocrinology 2000, 141, 1245–1253. [Google Scholar] [PubMed]
- Tollner, T.L.; Venners, S.A.; Hollox, E.J.; Yudin, A.I.; Liu, X.; Tang, G.; Xing, H.; Kays, R.J.; Lau, T.; Overstreet, J.W.; et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Tollner, T.L.; Yudin, A.I.; Treece, C.A.; Overstreet, J.W.; Cherr, G.N. Macaque sperm coating protein defb126 facilitates sperm penetration of cervical mucus. Hum. Reprod. 2008, 23, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.I.; Tollner, T.L.; Li, M.W.; Treece, C.A.; Overstreet, J.W.; Cherr, G.N. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol. Reprod. 2003, 69, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hamil, K.G.; Sivashanmugam, P.; Grossman, G.; Soundararajan, R.; Rao, A.J.; Richardson, R.T.; Zhang, Y.L.; O’Rand, M.G.; Petrusz, P.; et al. Primate epididymis-specific proteins: Characterization of ESC42, a novel protein containing a trefoil-like motif in monkey and human. Endocrinology 2001, 142, 4529–4539. [Google Scholar] [CrossRef] [PubMed]
- Yenugu, S.; Hamil, K.G.; Radhakrishnan, Y.; French, F.S.; Hall, S.H. The androgen-regulated epididymal sperm-binding protein, human beta-defensin 118 (defb118) (formerly esc42), is an antimicrobial beta-defensin. Endocrinology 2004, 145, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dong, J.; Gu, Y.; Liu, H.; Xin, A.; Shi, H.; Sun, F.; Zhang, Y.; Lin, D.; Diao, H. The novel human beta-defensin 114 regulates lipopolysaccharide (LPS)-mediated inflammation and protects sperm from motility loss. J. Biol. Chem. 2013, 288, 12270–12282. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.; Fok, K.L.; Chen, H.; Yu, M.K.; Duan, Y.; Chung, C.M.; Li, Z.; Wu, H.; Li, Z.; Zhang, H.; et al. Deficient human beta-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chan, H.C.; He, B.; So, S.C.; Chung, Y.W.; Shang, Q.; Zhang, Y.D.; Zhang, Y.L. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001, 291, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Zhang, Y.L.; Xiao, L.; Zheng, M.; Leung, K.M.; Chan, M.Y.; Lo, P.S.; Tsang, L.L.; Wong, H.Y.; Ho, L.S.; et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 2004, 6, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Yu, H.G.; Sun, F.; Zhang, Y.L.; Tanphaichitr, N. Rat recombinant beta-defensin 22 is a heparin-binding protein with antimicrobial activity. Asian J. Androl. 2011, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lushnikova, T.; Wang, G. Small lipopeptides possess anti-biofilm capability comparable to daptomycin and vancomycin. RSC Adv. 2015, 5, 59758–59769. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Makovitzki, A.; Avrahami, D.; Shai, Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar] [CrossRef] [PubMed]






| Sexually Transmitted Infection | Vaginitis | Urinary Tract Infection |
|---|---|---|
Viral Infection
| Yeast Infection
| Yeast Infection
|
| Peptide Name APD 1 ID UniProt ID PDB ID | Source | Microbicidal/Biological/Biochemical Properties | References for Spermicidal Effects | Remarks |
|---|---|---|---|---|
| LL-37 APD: AP00310 UniProt: P49913 PDB: 2K6O | Neutrophils, monocytes, lymphocytes, keratinocytes, epithelial cells of the lung, nasal cavity, genitourinary tract, gastrointestinal tract, ocular surface, and gingiva of Homo sapiens, Expression of LL-37 in Pan troglodytes (chimpanzee), Macaca mulatta (rhesus macaque) have also been described. | LL-37 exerts microbicidal effects on Gram positive and Gram negative bacteria, yeasts, Candida albicans, and HIV and other viruses, including those that cause STIs (see Table 4). In an aqueous solution, the structure of LL-37 is disordered. However, when it interacts with lipid membranes such as SDS and dodecylphosphocholine micelles, LL-37 adopts an alpha helical amphipathic structure, as revealed by NMR analysis with one side of the helix enriched in hydrophilic amino acids and the other side hydrophobic residues [77,78]. | [79] | LL-37 completely inhibits human and mouse sperm motility within 5 min at 10.8 μM and 3.7 μM, respectively. This inhibition is likely due to the specific disruptive effects of LL-37 on sperm surface membranes, as shown by electron microscopy and Sytox Green (a membrane impermeable DNA dye) staining. In addition, LL-37 treated sperm become prematurely acrosome reacted, thus hindering them to effectively bind to the egg. These specific adverse effects of LL-37 on sperm are likely due to its affinity for the negatively charged sulfogalactosylglycerolipid (SGG) present selectively on the sperm surface. The contraceptive effect of LL-37 is further demonstrated in female mice. Females, naturally cycling to the estrous phase and transcervically injected with sperm + LL-37, fail to become pregnant, whereas pregnancy occurs in 92% of females injected with sperm alone. The reproductive tract tissues of the females administered with LL-37 appears to be normal like that observed in females unexposed to LL-37 [79]. |
| Maximin 1 APD:AP00058 UniProt: P83080 PDB:None and Maximin 3 APD:AP00060 UniProt: P83082 PDB:None | Skin: Chinese red belly toad Bombina maxima | Lai et al. [80] have shown that maximin 1 and maximin 3 have microbicidal activities against both Gram positive and Gram negative bacteria and yeasts, Candida albicans. Both also have anti-cancer and anti-HIV activities. Although both maximin 1 and maximin 4 have no PDB ID, they are close homologs of maximin 4 (PDB: 2MHW). The 3D structure of maximin 4, as revealed by NMR analyses, is a linear cationic amphipathic peptide in solution, but forms a kinked alpha helix with lipid micelles [81]. | [80] | The spermicidal work is based only on sperm immotility. At 100 μg/mL (37 μM) of maximin 1 or maximin 3, ~80% of human sperm become immotile. |
| Magainin 2 APD:AP00144 UniProt: P11006 for magainins; magainin 2 is one of the five cleaved products of magainins. PDB: 2MAG | Skin and stomach: African clawed frog, Xenopus laevis | Magainin 2 has microbicidal effects on Gram positive and Gram negative bacteria, yeasts and viruses. It also acts against certain protozoa including malaria causing Plasmodium falciparum. However, magainin 2 does not have an anti-HIV activity [82]. NMR analysis indicates that magainin 2 adopts an alpha helical structure in the presence of SDS and other lipid micelles [83]. | [84,85,86,87] | Magainin A, a synthetic derivatives of magainin 2 [88] possess sperm immobilizing activity, as shown in rat, rabbit, monkey and human sperm. At ~50 μg/mL (20 μM), magainin A completely inhibit human/monkey sperm motility within 7–10 min of treatment, although it takes ~300 μg/mL (120 μM) of the peptide for the immediate immobilization of human sperm. Lower doses are required for rat and rabbit sperm for the same results [82,85,86]. These spermistatic effects are likely due to the ability of magainin A to disrupt the sperm surface membranes [85]. Magainin 2-amide (250 μg/mL (101 μM)) also exerts spermistatic effects on human sperm, although it is at 50% efficacy. Only when sperm are treated with both cyclodextrin and magainin 2-amide, sperm immotility is enhanced to 80%. Reddy’s group have demonstrated vaginal contraceptive effects of magainin A in rabbits and monkeys. Females each administered with 1 mg magainin A did not become pregnant upon natural mating. Side effects of magainin A on the female reproductive tract appeared to be minimum [82,84]. |
| Dermaseptin S1 APD:AP00157 UniProt: 80277 PDB:None | Skin: Sauvage’s leaf frog, Phyllomedusa sauvagii | Dermaseptin S1 has microbicidal activities against Gram positive and Gram negative bacteria and herpes simplex virus. It also kills the protozoa, Leishmania. Dermaseptin S1 has an alpha helix structure as revealed by circular dichroism analyses [89]. | [90] | The spermicidal work is based only on sperm immotility. Human sperm become completely immotile immediately after treatment with 200 μg/mL (58 μM) of dermaseptin S1. |
| Dermaseptin S4 APD:AP00160 UniProt: P80280 PDB: 2DD6 for a close analog of truncated dermaseptin-S4 (aa1-13). | Skin:Sauvage′s leaf frog, Phyllomedusa sauvagii | Dermaseptin S4 has microbicidal activities against Gram positive and Gram negative bacteria and viruses (herpes simplex virus and HIV). It also kills P falciparum protozoa. NMR analyses of a close analog of truncated dermaseptin S4 (aa1-13) indicates its alpha helix structure when interacting with lipid micelles [91]. | [90,92,93] | Despite a similar structure to dermaseptin S1, only 100 μg/mL (36 μM) of dermaseptin S4 is required to induce complete human sperm motility, indicating a twice spermistatic potency of dermaseptin S4 [90]. A higher spermistatic effect is further observed in a dermaseptin S4 derivative with one amino acid replacement with lysine to increase the positive charge. Only 20 μg/mL (7.2 μM) of this derivative is required to induce complete sperm immotility effects. The native dermaseptin S4 (100 μg/mL (36 μM)) and its derivative (20 μg/mL (7.2 μM)) cause 100% and 50% cytotoxicity to HeLa cells, respectively [92]. |
| Sarcotoxin Pd APD:AP02212 UniProt: None for this sarcotoxin but available for other sarcotoxin isoforms. PDB:None | Insects: rove beetles, Paederus dermatitis | Sarcotoxin Pd has microbicidal effects on Gram positive and Gram negative bacteria. Although there is no PDB information on sarcotoxin Pd, in the publication of Zare-Zardini et al. [94], a 3D structure of sarcotoxin Pd was shown to consist of two alpha helices. This structural information was obtained from computational modeling, although no details were given on how this modeling was performed. | [94] | The spermicidal work is based only on sperm immotility. The concentration of sarcotoxin Pd to immobilize human sperm is 80 μg/mL (22 μM). Cytotoxicity MTT assay was done on HeLa cells; at 80 μg/mL of sarcotoxin Pd, close to 100% of cells show cytotoxicity. |
| Nisin A APD:AP00205 UniProt:P13068 PDB: 1WCO | Bacteriocin from lactic acid bacteria (LAB), Lactococcus lactis (formerly called Streptococcus lactis) | The microbicidal effects of nisin A are more preferential to Gram positive bacteria; this may be attributed to its ability to bind to lipid II, a structural component of Gram positive bacterial peptidoglycans. The interaction between nisin A and lipid II leads to inhibition of the bacterial cell wall synthesis [95]. It is a cationic amphipathic lantipeptide (i.e., containing a lanthionine ether linkage between S3-C7 and 4 methyllanthionines: T8-C11, T13-C19, T23-C26 and T25-C28) (see Table 3). These thioether linkages create a constraint polycyclic feature to the peptide. However, NMR analysis revealed that nisin A is still flexible enough to interact with SDS micelles, first via ionic interaction and then through hydrophobic interaction. It was therefore proposed that nisin A can create pores in lipid bilayers following its tran-bilayer insertion [96,97]. | [98,99,100,101,102,103] | The sperm immobilizing effects of nisin A have been shown in various species, i.e., rats, rabbits, bulls, horses/ponies, boars, monkeys and humans [98,99,101]. The instantaneous spermistatic concentration of nisin A is 300–400 μg/mL (86–114 μM) for human/monkey sperm, 200 μg/mL (57 μM) for rabbit sperm and 50 μg/mL (14 μM) for rat sperm. Scanning electron microscopy revealed obvious disruption of the human sperm plasma membrane following treatment with 360 μg/mL (103 μM) of nisin A. This disruption was similar to what observed on the surface of Staphylococcus aureus bacteria, which were treated with a similar nisin A concentration. In contrast, human red blood cells were not affected by treatment with equivalent concentrations of nisin A. The preferential effects of nisin A on sperm plasma membrane permeabilization was also confirmed by propidium iodide nuclear staining of the treated sperm [102] Reddy et al. have further demonstrated that nisin A is an effective vaginal contraceptive in rats and rabbits. Female rats naturally cycling in the proestrous/estrous phase and vaginally administered with nisin A (200 μg each) did not become pregnant following natural mating [98]. Successful contraceptive results were likewise obtained in female rabbits intravaginally injected with nisin A (1 mg each), provided that mating took place within 30 min of the peptide administration [99]. In both animal species, the authors claimed that there were no changes to the anatomy of the female reproductive tract tissues or cytokine production profile in the females vaginally administered with nisin A at the contraceptive dose or even higher and repeated doses of nisin A [98,99,100]. By MTT assay, HeLa cells appeared to be less susceptible to cytotoxic effects of nisin A, as compared with sperm. |
| Pediocin CP2 APD:AP00634 UniProt: Q8RL96 PDB:None | Bacteriocin from LAB, Pediooccus acidilactici | Pediocin CP2 exerts microbicidal effects on both Gram positive and Gram negative bacteria as well as yeasts, Candida albicans [104] No PDB ID is available. However, the 3-D structure of its homolog, sakacin P (PDB: 1OG7, 68% identical peptide sequence to pediocin CP), reveals the presence of an alpha helix in the middle region of the peptide. | [104] | The spermicidal work is based only on sperm immotility. The concentration of pediocin CP2 to immobilize human sperm is >250 μg/mL (54 μM). There is no reported work on the cytoxicity concentration of pediocin CP2 on female reproductive tract tissues/cells. |
| Gramicidin A APD:AP00499 UniProt: None PDB: 1MAG | Bacteriocin from soil bacterium, Bacillus brevis | Gramicidin A exerts microbicidal effects on both Gram positive and Gram negative bacteria as well as viruses (including HIV) [105]. Gramicidin A has a special β-helix structure because of its possession of d-amino acids. Gramicidin A dimerizes with the N-terminus of each peptide being adjacent to each other. As a result, the dimer forms a cation channel in lipid bilayers with the two C-termini exposed [64]. | [105,106,107] | Gramicidin has been used for a long time in Russia as a spermicide as referred to in Bourinbaiar et al. [105], although detailed data of its efficacy is not available. Experimentally, the spermicidal effects of gramicidin A are based on human sperm immotility and consequently the inability of sperm to penetrate lamb cervical mucus [106]. Only 5 μg/mL (2.8 μM) of gramicidin A could completely immobilize sperm. Gramicidin D (mixture of gramicidin A, B and C) has similar sperm immobilizing effects but at a higher concentration than gramicidin A. |
| Subtilosin A APD:AP00928 UniProt:007623 PDB: 1PXQ | Bacteriocin from Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus atrophaeus | Subtilosin A has microbicidal effects on both Gram positive and Gram negative bacteria as well as herpes simplex viruses. It is a lantipeptide, containing a high percentage of hydrophobic residues (60%) with an overall negative charge of -2. Lanthionines between C13 and F22, C7 andT28, and C4 and F31 make the N-terminus and C-terminus fold towards each other, giving an overall boat conformation, as revealed by NMR analysis. There is an alpha helix in the C-terminus starting from G29 to W34 [108]. | [101,109] | The spermicidal test is based only on sperm immotility. At a single concentration tested, 800 μg/mL (233 μM), subtilosin A instantaneously immobilized bull and horse/pony sperm. The same spermicidal effects were observed in boar and rat sperm at 200 μg/mL (58 μM) of subtilosin A [101]. Human sperm treated with subtilosin also became immotile in a dose-dependent manner with complete immobilization at 110 μg/mL (32 μM). Notably, subtilosin at this concentration does not reduce cell viability in the human EpiVaginal ectocervical tissue model [109]. |
| Lacticin 3147 APD:AP01194 UniProt: 087236 PDB:None | Bacteriocin from LAB Lactococcus lactis DPC3147 | Lacticin 3147 is composed of two lantipeptide components, LtnA1 and LtnA2. Microbicidal activity of lacticin 3147 is preferential on Gram positive bacteria and is stronger with LtnA1 and LtnA2 combined, compared with each lacticin 3147 chain alone. There are four lanthionines in LtnA1 and two in LtnA2, which generate a polycyclic structure to both peptides. NMR analyses also reveal a helical structure in LtnA2 [110]. Note that there are a few D-Ala residues in both lantipeptide chains, and their existence is important for the microbicidal activity of lacticin [110]. | [101] | The spermicidal test is based only on sperm immotility. LtnA1 chain had much less spermicidal effects than LtnA2. The combination of LtnA1 + LtnA2 at 200 μg/mL (31 μM) could effectively immobilize rat, bull, and horse/pony sperm. But only 50 μg/mL (7.8 μM) of LtnA1 + LtnA2 was required to induce immotility of bull sperm [101]. |
 |
| Microbes 1 | Bacteria Gram +/− (B+/−) Virus (V) Yeast (Y) | References: Concentration 2 |
|---|---|---|
| Adenovirus (Ad) | V | Gordon et al. [160]: 111 μM |
| Acinetobacter baumannii | B− | Moffatt et al. [171]: 1.1 μM |
| Garcia-Quintanilla et al. [172]: 0.67 μM | ||
| Actinobacillus | B− | Ouhara et al. [173]: ~0.26–0.52 μM |
| Actinobacillus actinomycetemcomitans | B− | Tanaka et al. [174]: ~2.2–2.7 |
| Actinobacillus actinomycetemcomitans | B− | Ouhara et al. [173]: 2.2 μM |
| Bacillus anthracis | B− | Thwaite et al. [175]: 22 μM |
| Borrelia burgdorferi | Not applicable 3 | Lusitani et al. [176]: 8.8 μM |
| Borrelia spp | Not applicable 3 | Sambri et al. [169]: 100 μM |
| Burkholderia pseudomallei | B− | Kanthawong et al. [177]: 100 μM |
| Burkholderia thailandensis | B− | Kanthawong et al. [178]: 100 μM |
| Candida albicans | Y | Tsai et al. [179,180]: 8.9 μM |
| Capnocytophaga gingivalis | B− | Tanaka et al. [174]: 2.0 μM |
| Capnocytophaga ochracea | B− | Tanaka et al. [174]: 2.4 μM |
| Chlamydia trachomatis | B− | Tang et al. [181]: 20 μM |
| Clostridium difficile | B+ | McQuade et al. [182]: 10.7 μM |
| Enterococcus faecalis | B+ | Leszczynska et al. [183]: 12.5 μM |
| Escherichia coli | B− | Benincasa et al. [184]: 5 μM |
| Smeianov et al. [168]: 25 μM | ||
| Leszczynska et al. [185]: 5.6 μM | ||
| Chen et al. [186]: 0.07 μM | ||
| Kai-Larsen et al. [187]: 20 μM | ||
| Nagaoka et al. [188]: ~1-2 μM | ||
| Fusobacterium nucleatum | B− | Ouhara et al. [173]: 0.22 μM |
| Leszczynska et al. [183]: 49.8 μM | ||
| Haemophilus influenzae | B− | Leszczynska et al. [183]: 12.5 μM |
| Lysenko et al. [189]: 2.2 μM | ||
| Helicobacter pylori | B− | Leszczynska et al. [183]: 6.2 μM |
| Leszczynska et al. [185]: 2.2 μM | ||
| Herpes simplex virus type 1 | V | Gordon et al. [160]: 111 μM |
| HIV-1 | V | Wang et al. [135]: 1.6 μM |
| Bergman et al. [134]: 11.1 μM | ||
| Influenza A virus (IAV) | V | Tripathi et al. [190]: 13 μM |
| Barlow et al. [191]: 11.1 μM | ||
| Tripathi et al. [192]: 6.7 μM | ||
| Klebsiella pneumoniae | B− | De Majumdar et al. [193]: >11.1 μM |
| Moraxella catarrhalis | B− | Leszczynska et al. [183]: 6.2 μM |
| Neisseria gonorrhoeae | B− | Bergman et al. [194]: 0.8 μM |
| Neisseria meningitidis | B− | Leszczynska et al. [183]: 12.5 μM for strain B |
| Leszczynska et al. [183]: 24.9 μM for strain C | ||
| Jones et al. [195]: 10 μM | ||
| Peptostreptococcus anaerobius | B+ | Leszczynska et al. [183]: 49.8 μM |
| Porphyromonas gingivalis | B− | Leszczynska et al. [183]: 49.8 μM |
| Ouhara et al. [173]: 11.1 μM | ||
| Prevotella intermedia | B− | Ouhara et al. [173]: 1.1 μM |
| Pseudomonas aeruginosa | B− | Bergsson et al. [196]: 5.6 μM |
| Dean et al. [197]: 0.22 μM | ||
| Dosler and Karaaslan [198]: ~14.2–28.4 μM | ||
| Gordon et al. [160]: ~11.1–22.2 μM | ||
| Leszczynska et al. [183]: 99.7 μM | ||
| Respiratory syncytial virus | V | Currie et al. [199]: 5.6 μM |
| Staphylococcus aureus | B+ | Leszczynska et al. [183]: 6.2 μM |
| Noore et al. [200]: 2 μM | ||
| Chen et al. [186]: 0.67 μM | ||
| Senyurek et al. [201]: 11.1 μM | ||
| Nagaoka et al. [188]: 1 μM | ||
| Gordon et al. [160]: ~11.1–22.2 μM | ||
| Staphylococcus epidermidis | B+ | Leszczynska et al. [183]: 12.5 μM |
| Gordon et al. [160]: ~11.1–22.2 μM | ||
| Streptococcus mitis | B+ | Ouhara et al. [173]: 2.2 μM |
| Streptococcus mutans | B+ | Ouhara et al. [173]: 0.22 μM |
| Leszczynska et al. [183]: 6.2 μM | ||
| Streptococcus pneumoniae | B+ | Nagaoka et al. [188]: 1 μM |
| Leszczynska et al. [183]: 3.1 μM | ||
| Streptococcus pyogenes | B+ | Leszczynska et al. [183]: 3.1 μM |
| Streptococcus salivarius | B+ | Ouhara et al. [173]: 1.1 μM |
| Leszczynska et al. [183]: 6.2 μM | ||
| Streptococcus sanguis | B+ | Ouhara et al. [173]: 0.22 μM |
| Leszczynska et al. [183]: 6.2 μM | ||
| Streptococcus sobrinus | B+ | Ouhara et al. [173]: 1.1 μM |
| Tannerella forsythensis | B+ | Leszczynska et al. [183]: 49.8 μM |
| Treponema pallidum | B- | Sambri et al. [169]: 100.1 μM |
| Ureaplasma parvum | NA 3 | Xiao et al. [202]: 22.2 μM |
| Ureaplasma urealyticum | NA 3 | Xiao et al. [202]: 22.2 μM |
| Vaccinia virus | V | Howell et al. [203]: 20 μM |
| Varicella zoster virus (VZV) | V | Crack et al. [204]: 0.1 μM |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanphaichitr, N.; Srakaew, N.; Alonzi, R.; Kiattiburut, W.; Kongmanas, K.; Zhi, R.; Li, W.; Baker, M.; Wang, G.; Hickling, D. Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharmaceuticals 2016, 9, 13. https://doi.org/10.3390/ph9010013
Tanphaichitr N, Srakaew N, Alonzi R, Kiattiburut W, Kongmanas K, Zhi R, Li W, Baker M, Wang G, Hickling D. Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharmaceuticals. 2016; 9(1):13. https://doi.org/10.3390/ph9010013
Chicago/Turabian StyleTanphaichitr, Nongnuj, Nopparat Srakaew, Rhea Alonzi, Wongsakorn Kiattiburut, Kessiri Kongmanas, Ruina Zhi, Weihua Li, Mark Baker, Guanshun Wang, and Duane Hickling. 2016. "Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides" Pharmaceuticals 9, no. 1: 13. https://doi.org/10.3390/ph9010013
APA StyleTanphaichitr, N., Srakaew, N., Alonzi, R., Kiattiburut, W., Kongmanas, K., Zhi, R., Li, W., Baker, M., Wang, G., & Hickling, D. (2016). Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharmaceuticals, 9(1), 13. https://doi.org/10.3390/ph9010013