Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization of Bromelain Extracts
2.2. Cytotoxic Effects of Bromelain Extracts on RAW264.7 Cells
2.3. Effects of Bromelain Extracts on LPS-Induced NO Production and Expressions of iNOS and COX-2 in RAW264.7 Cells
2.4. Effects of Bromelain Extracts on LPS-Induced IL-6 and TNF-α in RAW264.7 Cells
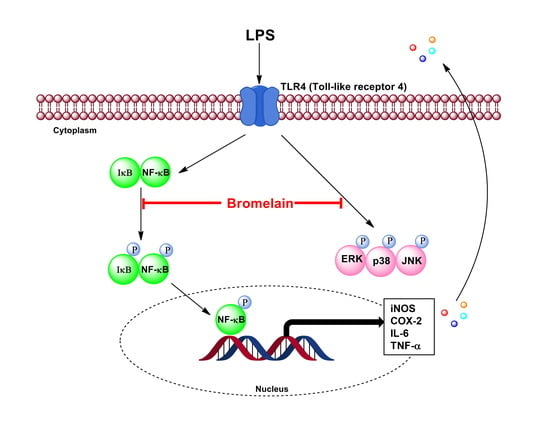
2.5. Effects of Bromelain Extracts on LPS-Induced Expression of NF-κB Pathway-Related Proteins
2.6. Effects of Bromelain Extracts on LPS-Induced Phosphorylation of the MAPKs Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Bromelain Extracts from Pineapple Rhizome
4.3. Characterization of Bromelain Extracts
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Measurement of Nitric oxide and Cytokines Productions
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharyya, B.K. Bromelain: An overview. Nat. Prod. Radiance 2008, 7, 359–363. [Google Scholar]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tochi, B.N.; Wang, Z.; Xu, S.-Y.; Zhang, W. Therapeutic application of pineapple protease (bromelain): A review. Pak. J. Nutr. 2008, 7, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell. Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Tysnes, B.B.; Maurert, H.R.; Porwol, T.; Probst, B.; Bjerkvig, R.; Hoover, F. Bromelain reversibly inhibits invasive properties of glioma cells. Neoplasia 2001, 3, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhui, K.; Prasad, S.; George, J.; Shukla, Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009, 282, 167–176. [Google Scholar] [CrossRef]
- Baez, R.; Lopes, M.T.; Salas, C.E.; Hernandez, M. In Vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Med. 2007, 73, 1377–1383. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Manosroi, W.; Manosroi, J. Toxicity reduction and MMP-2 stimulation of papain and bromelain loaded in elastic niosomes. J. Biomed. Nanotechnol. 2012, 8, 720–729. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Vivithanaporn, P.; Unchern, S. Anti-inflammatory effects of bromelain in Lps-induced human U937 macrophages. Chiang Mai J. Sci. 2018, 45, 299–307. [Google Scholar]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Mosser, D.M.; Gonçalves, R. Activation of murine macrophages. Curr. Protoc. Immunol. 2015, 111, 14.12.1–14.12.8. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, P.H.; von Känel, R. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [Green Version]
- Sharma, L. The role of varus and valgus alignment in knee osteoarthritis. Arthritis Rheum. 2007, 56, 1044–1047. [Google Scholar] [CrossRef]
- Prescott, S.M.; Fitzpatrick, F. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta 2000, 1470, M69–M78. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Manzoor, Z.; Koh, Y.-S. Mitogen-activated protein kinases in inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Cameron, N.E.; Cotter, M.A. Pro-inflammatory mechanisms in diabetic neuropathy: Focus on the nuclear factor kappa B pathway. Curr. Drug Targets 2008, 9, 60–67. [Google Scholar] [CrossRef]
- Akira, S.; Hoshino, K. Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J. Infect. Dis. 2003, 187, S356–S363. [Google Scholar] [CrossRef]
- Biswas, S.K.; Tergaonkar, V. Myeloid differentiation factor 88-independent Toll-like receptor pathway: Sustaining inflammation or promoting tolerance? Int. J. Biochem. Cell Biol. 2007, 39, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Wilde, G.; Notebaert, S.; Berghe, W.V.; Haegeman, G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem. Pharmacol. 2002, 64, 963–970. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappa B signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Schmitz, M.L. Of mice and men: Meeting on the biology and pathology of NF-κB. EMBO Rep. 2004, 5, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [Green Version]
- Thalhamer, T.; McGrath, M.; Harnett, M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Heinsbroek, S.E.; Gordon, S. The role of macrophages in inflammatory bowel diseases. Expert Rev. Mol. Med. 2009, 11, e14. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.S.; Chong, Y.H.; Kang, J.L. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-κB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology 2006, 225, 36–47. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Ooppachai, C.; Pompimon, W.; Limtrakul, P. O-methylbulbocapnine and dicentrine suppress LPS-induced inflammatory response by blocking NF-κB and AP-1 activation through inhibiting MAPKs and Akt signaling in RAW264. 7 macrophages. Biol. Pharm. Bull. 2018, 41, 1219–1227. [Google Scholar] [CrossRef] [Green Version]
- Moynagh, P.N. The NF-κB pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T.; Bebien, M.; Liu, G.Y.; Nizet, V.; Karin, M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 2005, 434, 1138–1143. [Google Scholar] [CrossRef]
- Hori, M.; Kita, M.; Torihashi, S.; Miyamoto, S.; Won, K.-J.; Sato, K.; Ozaki, H.; Karaki, H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol.-Gastr. Liver Physiol. 2001, 280, G930–G938. [Google Scholar] [CrossRef]
- Desser, L.; Rehberger, A.; Paukovits, W. Proteolytic enzymes and amylase induce cytokine production in human peripheral blood mononuclear cells in vitro. Cancer Biother. Radiopharm. 1994, 9, 253–263. [Google Scholar] [CrossRef]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Mynott, T.L.; Ladhams, A.; Scarmato, P.; Engwerda, C.R. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J. Immunol. 1999, 163, 2568–2575. [Google Scholar] [PubMed]
- Liu, H.-S.; Pan, C.-E.; Liu, Q.-G.; Yang, W.; Liu, X.-M. Effect of NF-κB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J. Gastroenterol. 2003, 9, 2513. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Q.; Malcolm, K.; Worthen, G.S.; Gardai, S.; Schiemann, W.P.; Fadok, V.A.; Bratton, D.L.; Henson, P.M. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-β. J. Biol. Chem. 2002, 277, 14884–14893. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-κB: A sensor for smoke and stress signals. Annals N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef]
- Potoyan, D.A.; Zheng, W.; Komives, E.A.; Wolynes, P.G. Molecular stripping in the NF-κB/IκB/DNA genetic regulatory network. Proc. Natl. Acad. Sci. USA 2016, 113, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584191–584196. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 267–275. [Google Scholar] [CrossRef]
- Ketnawa, S.; Sai-Ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of bromelain from pineapple peel (Nang Lae cultv) by aqueous two phase system. Asian J. Food Agro-Ind. 2009, 2, 457–468. [Google Scholar]
- LaemmLi, U.K. Cleavage of structural proteins during the assembly of the head ofbacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insuan, O.; Janchai, P.; Thongchuai, B.; Chaiwongsa, R.; Khamchun, S.; Saoin, S.; Insuan, W.; Pothacharoen, P.; Apiwatanapiwat, W.; Boondaeng, A.; et al. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Curr. Issues Mol. Biol. 2021, 43, 93-106. https://doi.org/10.3390/cimb43010008
Insuan O, Janchai P, Thongchuai B, Chaiwongsa R, Khamchun S, Saoin S, Insuan W, Pothacharoen P, Apiwatanapiwat W, Boondaeng A, et al. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Current Issues in Molecular Biology. 2021; 43(1):93-106. https://doi.org/10.3390/cimb43010008
Chicago/Turabian StyleInsuan, Orapin, Phornphimon Janchai, Benchaluk Thongchuai, Rujirek Chaiwongsa, Supaporn Khamchun, Somphot Saoin, Wimonrut Insuan, Peraphan Pothacharoen, Waraporn Apiwatanapiwat, Antika Boondaeng, and et al. 2021. "Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells" Current Issues in Molecular Biology 43, no. 1: 93-106. https://doi.org/10.3390/cimb43010008
APA StyleInsuan, O., Janchai, P., Thongchuai, B., Chaiwongsa, R., Khamchun, S., Saoin, S., Insuan, W., Pothacharoen, P., Apiwatanapiwat, W., Boondaeng, A., & Vaithanomsat, P. (2021). Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-B- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Current Issues in Molecular Biology, 43(1), 93-106. https://doi.org/10.3390/cimb43010008