Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases
Abstract
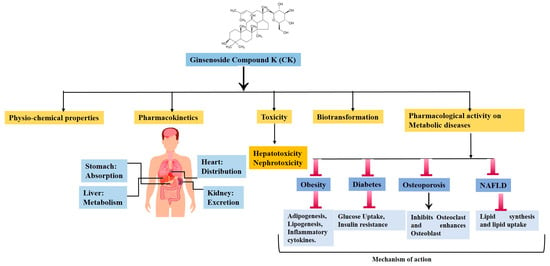
:1. Introduction
2. Physical and Chemical Properties
3. Pharmacokinetics, Safety, and Toxicological Studies of CK and Its Derivatives
| Study Model | Concentrations | Method of Detection | Duration of Experiment | Result | Ref. |
|---|---|---|---|---|---|
| 3T3-L1 preadipocyte cell lines | (0, 10, 20, 30, and 40 μM) | MTS assay | 24 h | A high dose of CK (40 μM) did not affect cell viability. | [27] |
| HaCaT keratinocytes cells | 0.2, 0.4, 0.6, 0.8, 1.0, and 10.0 μM | MTT assay | 24 h | Below 10 μM showed safe survival of HaCaT cells. | [28] |
| MC3T3-E1 osteoblastic cell line | 0.01, 0.1, 1, and 10 μM | MTT assay | 48 h | No cytotoxicity was observed. | [8] |
| HepG2 | 1, 2, 5, 10, 15, 20, and 30 μM | MTT assay | 24 h | Cell viability reduces with increasing concentrations. | [29] |
| HepG2 | 5−40 μM | CellTiter 96 AQueous One Solution Cell Proliferation Assay kit | 24 h | CK did not exhibit any cellular toxicity below 40 μM. | [30] |
| HT22 mouse hippocampal neuron cell | 2.5, 5, and 10 μM | MTT assay | 24 h | CK can increase the survival of HT22 cells. | [31] |
| L02 Human liver cell line | 0.625, 1.25, 2.5, 5, 10, and 20 μM | MTT assay | 24 h | The cell viability appeared at the dosages of 1.25–10 μM. | [32] |
| RA-FLS and Raw 264.7 | 0.1, 0.5, 2.5, and 5 μM | MTT assay | 48 h | The survival rate of both cells was not impacted at doses of ≤5 μM. | [33] |
| MIN6 cell line | 2, 4, 8, 16, and 32 μM | MTT assay | 24 h | At 16 μM CK showed little toxicity on MIN6 cell | [34] |
| Hk-1 Nasopharyngeal Carcinoma cells, | 1–20 μM | MTT assay | 24 h | The IC50 of CK was 11.5 on HK-1 cells | [35] |
| A549 lung cancer cells, MCF7 breast cancer cells, Caco-2 human colorectal adenocarcinoma cells, and normal RAW 264.7 cells | 0, 3.125, 6.25, 12.5, and 25 μg/mL | MTT assay | 24 h | At 12.5 μg/mL concentration, CK showed considerable cytotoxic effect on A549 cells, MCF-7 cells, and Caco-2 cells growth. However, at 6.25 μg/mL, Raw 264.7 cells showed less toxicity. | [36] |
| HT-29 Human colon cancer cells | 8, 16, 32, and 64 μmol/L | MTT assay | 24 h | CK inhibited the growth of HT-29 cells in a dose-dependent manner. | [37] |
| HL-60 human myeloid leukemia cell line | 10, 20, 30, and 50 μM | MTT assay | 72–96 h | 24.3 μM was needed to achieve 50% growth inhibition (IC50) at 96 h. | [45] |
| U937, Jurkat, CEM-CM3, Molt4, and H9 leukemia cell lines | Did not mention | MTT assay | 96 h | The IC50 values of CK were as follows: 20 μg/mL for U937, 26 μg/mL for Jurkat, 36 μg/mL for CEM-CM3, 44 μg/mL for Molt 4, and 64 μg/mL for H9. | [46] |
| Rat and mice | 8 and 10 mg/kg, respectively | Acute oral repeated dose | 26 weeks | No indications of clinical harm or death after 14 days. A few variations were observed in this shift at weeks 9, 10, 12, 15, 17, 21–24, and 26. As a result, CK had a minimally negative impact on the animal’s body weight. | [38] |
| Beagle dogs | 4, 12, or 36 mg/kg | Oral doses | 26 weeks | No obvious toxicity was shown by the animals in the 4 and 12 mg/kg groups. The 36 mg/kg group showed elevated plasma enzyme levels, localized liver necrosis, and a decrease in body weight. | [47] |
4. Biotransformation of CK
4.1. Enzymatically Synthesis
4.2. Biotransformation of CK by Human Gut Microbiota
5. Mechanism of CK against Metabolic Diseases
5.1. Obesity
5.2. Diabetes and Related Complications
5.3. Osteoporosis
5.4. Non-Alcoholic Fatty Liver Disease (NAFLD)
6. Synthesis of CK Analogues and Their Pharmacological Activity
7. Discussion and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Lin, Z.-J.; Li, C.-C.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Li, F.; Yuan, L.-Q.; Li, Z.-H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A. Strengthening the International Diabetes Federation (IDF). Diabetes Res. Clin. Pract. 2020, 160, 108029. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Perumalsamy, H.; Markus, J.; Balusamy, S.R.; Wang, C.; Ho Kang, S.; Lee, S.; Park, S.Y.; Kim, S.; Castro-Aceituno, V. Development of Lactobacillus kimchicus DCY51T-mediated gold nanoparticles for delivery of ginsenoside compound K: In vitro photothermal effects and apoptosis detection in cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Ahn, J.C.; Mathiyalagan, R.; Rupa, E.J.; Akter, R.; Karim, M.R.; Jung, D.H.; Yang, D.U.; Yang, D.C.; Jung, S.K. Antioxidant Activity of Panax ginseng to Regulate ROS in Various Chronic Diseases. Appl. Sci. 2023, 13, 2893. [Google Scholar] [CrossRef]
- Kang, S.; Siddiqi, M.H.; Yoon, S.J.; Ahn, S.; Noh, H.-Y.; Kumar, N.S.; Kim, Y.-J.; Yang, D.-C. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: An in silico and in vitro study. In Vitro Cell. Dev. Biol.—Anim. 2016, 52, 895–905. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, J.; Gu, Q.; Harindintwali, J.D.; Yu, X.; Liu, X. Combinatorial Enzymatic Catalysis for Bioproduction of Ginsenoside Compound K. J. Agric. Food Chem. 2023, 71, 3385–3397. [Google Scholar] [CrossRef]
- Chu, L.L.; Hanh, N.T.Y.; Quyen, M.L.; Nguyen, Q.H.; Lien, T.T.P.; Do, K.V. Compound K Production: Achievements and Perspectives. Life 2023, 13, 1565. [Google Scholar] [CrossRef]
- Yang, X.-D.; Yang, Y.-Y.; Ouyang, D.-S.; Yang, G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef]
- Han, G.C.; Ko, S.K.; Sung, J.H.; Chung, S.H. Compound K Enhances Insulin Secretion with Beneficial Metabolic Effects in db/db Mice. J. Agric. Food Chem. 2007, 55, 10641–10648. [Google Scholar] [CrossRef]
- Xu, J.; Dong, J.; Ding, H.; Wang, B.; Wang, Y.; Qiu, Z.; Yao, F. Ginsenoside compound K inhibits obesity-induced insulin resistance by regulation of macrophage recruitment and polarization via activating PPARγ. Food Funct. 2022, 13, 3561–3571. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Oh, D.H.; Choi, M.C.; Lee, S.Y.; Ahn, K.J.; Chung, H.Y.; Lim, S.J.; Chung, S.H.; Jeong, I.K. Compound K attenuates glucose intolerance and hepatic steatosis through AMPK-dependent pathways in type 2 diabetic OLETF rats. Korean J. Intern. Med. 2018, 33, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. Peroxisome Proliferator-Activated Receptor-γ as a Target and Regulator of Epigenetic Mechanisms in Nonalcoholic Fatty Liver Disease. Cells 2023, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yoon, M. Compound K, a novel ginsenoside metabolite, inhibits adipocyte differentiation in 3T3-L1 cells: Involvement of angiogenesis and MMPs. Biochem. Biophys. Res. Commun. 2012, 422, 263–267. [Google Scholar] [CrossRef]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, M.-H.; Jang, H.-M.; Im, W.-T.; Lee, J.; Kim, S.-H.; Jeon, G.J. A rare ginsenoside compound K (CK) induces apoptosis for breast cancer cells. J. Anim. Reprod. Biotechnol. 2023, 38, 167–176. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kim, E.; Chun, S. Ginsenoside compound K induces ros-mediated apoptosis and autophagic inhibition in human neuroblastoma cells in vitro and in vivo. Int. J. Mol. Sci. 2019, 20, 4279. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Wang, Y.; Yang, G.; Huang, J.; Tan, Z.; Wang, Y.; Zhou, G.; Liao, J.; Ouyang, D. Food and sex-related impacts on the pharmacokinetics of a single-dose of ginsenoside compound K in healthy subjects. Front. Pharmacol. 2017, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J. Ginseng Res. 2013, 37, 451. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, L.; Huang, J.; Wang, Y.; Yang, G.; Tan, Z.; Wang, Y.; Zhou, G.; Liao, J.; Ouyang, D. Single-and multiple-dose trials to determine the pharmacokinetics, safety, tolerability, and sex effect of oral ginsenoside compound K in healthy Chinese volunteers. Front. Pharmacol. 2018, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kang, J.-W.; Song, Y.-S.; Kim, J.-H.; Kim, M.S.; Bak, Y.; Oh, D.-K.; Yoon, D.-Y. Compound K attenuates lipid accumulation through down-regulation of peroxisome proliferator-activated receptor γ in 3T3-L1 cells. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 141–147. [Google Scholar] [CrossRef]
- Fang, C.; Yang, H.; Zhu, C.; Fan, D.; Deng, J. Ginsenoside CK inhibits androgenetic alopecia by regulating Wnt/β-catenin and p53 signaling pathways in AGA mice. Food Front. 2023, 4, 1270–1284. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Fan, D. Ginsenoside CK ameliorates hepatic lipid accumulation via activating the LKB1/AMPK pathway in vitro and in vivo. Food Funct. 2022, 13, 1153–1167. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yuan, H.D.; Chung, I.K.; Chung, S.H. Compound K, Intestinal Metabolite of Ginsenoside, Attenuates Hepatic Lipid Accumulation via AMPK Activation in Human Hepatoma Cells. J. Agric. Food Chem. 2009, 57, 1532–1537. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yang, Q.; Lan, X.; Wang, J.; Cao, Z.; Shi, X.; Li, J.; Kan, M.; Qu, X. Ginsenoside compound K ameliorates Alzheimer’s disease in HT22 cells by adjusting energy metabolism. Mol. Biol. Rep. 2019, 46, 5323–5332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zeng, X.; Rao, T.; Tan, Z.; Zhou, G.; Ouyang, D.; Chen, L. Evaluating the protective effects of individual or combined ginsenoside compound K and the downregulation of soluble epoxide hydrolase expression against sodium valproate-induced liver cell damage. Toxicol. Appl. Pharmacol. 2021, 422, 115555. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kang, E.H.; Lee, E.Y.; Gong, H.S.; Kang, H.S.; Shin, K.; Lee, E.B.; Song, Y.W.; Lee, Y.J. Joint-protective effects of compound K, a major ginsenoside metabolite, in rheumatoid arthritis: In vitro evidence. Rheumatol. Int. 2013, 33, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, W.; Xiao, D.; Wei, S.; Cui, W.; Chen, W.; Hu, Y.; Bi, X.; Kim, Y.; Li, J.; et al. Compound K, a final intestinal metabolite of ginsenosides, enhances insulin secretion in MIN6 pancreatic β-cells by upregulation of GLUT2. Fitoterapia 2013, 87, 84–88. [Google Scholar] [CrossRef]
- Law, C.K.-M.; Kwok, H.-H.; Poon, P.-Y.; Lau, C.-C.; Jiang, Z.-H.; Tai, W.C.-S.; Hsiao, W.W.-L.; Mak, N.-K.; Yue, P.Y.-K.; Wong, R.N.-S. Ginsenoside compound K induces apoptosis in nasopharyngeal carcinoma cells via activation of apoptosis-inducing factor. Chin. Med. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, V.; Nahar, J.; Murugesan, M.; Subramaniyam, S.; Kong, B.M.; Choi, S.-K.; Lee, C.-S.; Ling, L.; Yang, D.U.; Yang, D.C. In silico and in vitro inhibition of host-based viral entry targets and cytokine storm in COVID-19 by ginsenoside compound K. Heliyon 2023, 9, e19341. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kim, H.S.; Kim, D.H.; Bae, S.C.; Hyun, J.W. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. Int. J. Oncol. 2013, 43, 1907–1914. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, T.; Wang, G.; Li, G.; Sun, C.; Jiang, Z.; Yang, J.; Li, Y.; You, Y.; Wu, X.; et al. Preclinical safety of ginsenoside compound K: Acute, and 26-week oral toxicity studies in mice and rats. Food Chem. Toxicol. 2019, 131, 110578. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Ding, M.; Xin, Y.; Xuan, Y.; Zhao, Y. Genotoxicity and subchronic toxicological study of a novel ginsenoside derivative 25-OCH3-PPD in beagle dogs. J. Ginseng Res. 2019, 43, 562–571. [Google Scholar] [CrossRef]
- Igami, K.; Ozawa, M.; Inoue, S.; Iohara, D.; Miyazaki, T.; Shinoda, M.; Anraku, M.; Hirayama, F.; Uekama, K. The formation of an inclusion complex between a metabolite of ginsenoside, compound K and γ-cyclodextrin and its dissolution characteristics. J. Pharm. Pharmacol. 2016, 68, 646–654. [Google Scholar] [CrossRef]
- Song, I.-S.; Cha, J.-S.; Choi, M.-K. Characterization, in vivo and in vitro evaluation of solid dispersion of curcumin containing d-α-Tocopheryl polyethylene glycol 1000 succinate and mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Z.; Hou, J.; Jin, X.; Ke, Z.; Liu, D.; Du, M.; Jia, X.; Lv, H. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int. J. Nanomed. 2017, 2017, 7653–7667. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Yang, D.C. Bovine serum albumin as a nanocarrier for the efficient delivery of ginsenoside compound K: Preparation, physicochemical characterizations and in vitro biological studies. RSC Adv. 2017, 7, 15397–15407. [Google Scholar] [CrossRef]
- Kim, S.; Wang, R.; Dhandapani, S.; Kang, K.; Cho, I.-H.; Kim, Y.-J. Novel modified probiotic gold nanoparticles loaded with ginsenoside CK exerts an anti-inflammation effect via NF-κB/MAPK signaling pathways. Arab. J. Chem. 2024, 17, 105650. [Google Scholar] [CrossRef]
- Lee, S.-J.; Ko, W.-G.; Kim, J.-H.; Sung, J.-H.; Lee, S.-J.; Moon, C.-K.; Lee, B.-H. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem. Pharmacol. 2000, 60, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lim, H.K.; Kim, S.U.; Kim, Y.W.; Kim, W.T.; Chung, H.S.; Choo, M.K.; Kim, D.H.; Kim, H.S.; Shim, M.J. Induction of apoptosis by ginseng saponin metabolite in U937 human monocytic leukemia cells. J. Food Biochem. 2005, 29, 27–40. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Wang, T.; Wang, G.; Li, G.; Sun, C.; Lin, J.; Sun, L.; Sun, X.; Cho, S. Repeated-dose 26-week oral toxicity study of ginsenoside compound K in Beagle dogs. J. Ethnopharmacol. 2020, 248, 112323. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Park, K.-K.; Lee, J.-M.; Chun, K.-S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surh, Y.-J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000, 150, 41–48. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Li, J.; Fu, L.; Gao, J.; Du, X.; Bi, H.; Zhou, Y.; Tai, G. Highly selective biotransformation of ginsenoside Rb1 to Rd by the phytopathogenic fungus Cladosporium fulvum (syn. Fulvia fulva). J. Ind. Microbiol. Biotechnol. 2009, 36, 721–726. [Google Scholar] [CrossRef]
- Cao, S.; Yang, F.; Tian, F.; Liu, X.; Fan, D.; Wu, Z. Immobilized β-glucosidase on Cu(PTA) for the green production of rare ginsenosides CK. Process Biochem. 2023, 133, 169–178. [Google Scholar] [CrossRef]
- Tran, T.N.A.; Son, J.-S.; Awais, M.; Ko, J.-H.; Yang, D.C.; Jung, S.-K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering 2023, 10, 484. [Google Scholar]
- Duan, Z.; Zhu, C.; Shi, J.; Fan, D.; Deng, J.; Fu, R.; Huang, R.; Fan, C. High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase. Chin. J. Chem. Eng. 2018, 26, 1591–1597. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, X.-W.; Zhou, W.; Li, X.-W.; Feng, M.-Q.; Zhou, P. Purification and properties of a novel ${\beta} $-glucosidase, hydrolyzing ginsenoside Rb1 to CK, from Paecilomyces Bainier. J. Microbiol. Biotechnol. 2008, 18, 1081–1089. [Google Scholar] [PubMed]
- An, D.-S.; Cui, C.-H.; Lee, H.-G.; Wang, L.; Kim, S.C.; Lee, S.-T.; Jin, F.; Yu, H.; Chin, Y.-W.; Lee, H.-K. Identification and characterization of a novel Terrabacter ginsenosidimutans sp. nov. β-glucosidase that transforms ginsenoside Rb1 into the rare gypenosides XVII and LXXV. Appl. Environ. Microbiol. 2010, 76, 5827–5836. [Google Scholar] [CrossRef]
- Zhong, F.-L.; Dong, W.-W.; Wu, S.; Jiang, J.; Yang, D.-C.; Li, D.; Quan, L.-H. Biotransformation of gypenoside XVII to compound K by a recombinant β-glucosidase. Biotechnol. Lett. 2016, 38, 1187–1193. [Google Scholar] [CrossRef]
- Choi, J.-H.; Shin, K.-C.; Oh, D.-K. An L213A variant of β-glycosidase from Sulfolobus solfataricus with increased α-L-arabinofuranosidase activity converts ginsenoside Rc to compound K. PLoS ONE 2018, 13, e0191018. [Google Scholar] [CrossRef]
- Park, C.-S.; Yoo, M.-H.; Noh, K.-H.; Oh, D.-K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biotechnol. 2010, 87, 9–19. [Google Scholar] [CrossRef]
- Noh, K.-H.; Son, J.-W.; Kim, H.-J.; Oh, D.-K. Ginsenoside compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus. Biosci. Biotechnol. Biochem. 2009, 73, 316–321. [Google Scholar] [CrossRef]
- Yoo, M.-H.; Yeom, S.-J.; Park, C.-S.; Lee, K.-W.; Oh, D.-K. Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosu s. Appl. Microbiol. Biotechnol. 2011, 89, 1019–1028. [Google Scholar] [CrossRef]
- Quan, L.-H.; Jin, Y.; Wang, C.; Min, J.-W.; Kim, Y.-J.; Yang, D.-C. Enzymatic transformation of the major ginsenoside Rb2 to minor compound Y and compound K by a ginsenoside-hydrolyzing β-glycosidase from Microbacterium esteraromaticum. J. Ind. Microbiol. Biotechnol. 2012, 39, 1557–1562. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Li, X.; Tian, D.; Fan, D.; Ma, X.; Wu, Z. Co-immobilized β-glucosidase and snailase in green synthesized Zn-BTC for ginsenoside CK biocatalysis. Biochem. Eng. J. 2022, 188, 108677. [Google Scholar] [CrossRef]
- Cao, S.; Li, R.; Tian, F.; Liu, X.; Fan, D.; Wu, Z. Construction of a hollow MOF with high sedimentation performance and co-immobilization of multiple-enzymes for preparing rare ginsenoside CK. React. Chem. Eng. 2023, 8, 2804–2817. [Google Scholar] [CrossRef]
- Cui, L.; Wu, S.-Q.; Zhao, C.-A.; Yin, C.-R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J. Ginseng Res. 2016, 40, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, B.; Hu, X.; Zhang, H.; Jiang, B.; Spranger, M.I.; Zhao, Y. Transformation of Bioactive Compounds by Fusarium sacchari Fungus Isolated from the Soil-Cultivated Ginseng. J. Agric. Food Chem. 2007, 55, 9373–9379. [Google Scholar] [CrossRef]
- Quan, L.-H.; Kim, Y.-J.; Li, G.H.; Choi, K.-T.; Yang, D.-C. Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius. World J. Microbiol. Biotechnol. 2013, 29, 1001–1007. [Google Scholar] [CrossRef]
- Cheng, L.-Q.; Kim, M.K.; Lee, J.-W.; Lee, Y.-J.; Yang, D.-C. Conversion of major ginsenoside Rb 1 to ginsenoside F 2 by Caulobacter leidyia. Biotechnol. Lett. 2006, 28, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Sung, J.-H.; Matsumiya, S.; Uchiyama, M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Medica 1996, 62, 453–457. [Google Scholar] [CrossRef]
- Kim, K.-A.; Jung, I.-H.; Park, S.-H.; Ahn, Y.-T.; Huh, C.-S.; Kim, D.-H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE 2013, 8, e62409. [Google Scholar] [CrossRef]
- Zhou, W.; Feng, M.-Q.; Li, J.-Y.; Zhou, P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. J. Asian Nat. Prod. Res. 2006, 8, 519–527. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Q.; Li, J.Y.; Zhang, X.C.; Zhou, P. Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp. 229. J. Appl. Microbiol. 2008, 104, 699–706. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yan, M.; Sun, C.; Zheng, P. Screening of plant pathogenic fungi by ginsenoside compound K production. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2011, 36, 1596–1598. [Google Scholar]
- Chen, G.-T.; Yang, M.; Song, Y.; Lu, Z.-Q.; Zhang, J.-Q.; Huang, H.-L.; Wu, L.-J.; Guo, D.-A. Microbial transformation of ginsenoside Rb 1 by Acremonium strictum. Appl. Microbiol. Biotechnol. 2008, 77, 1345–1350. [Google Scholar] [CrossRef]
- Chi, H.; Ji, G.-E. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol. Lett. 2005, 27, 765–771. [Google Scholar] [CrossRef]
- Park, J.K.; Yang, D.U.; Arunkumar, L.; Han, Y.; Lee, S.J.; Arif, M.H.; Li, J.F.; Huo, Y.; Kang, J.P.; Hoang, V.A.; et al. Cumulative Production of Bioactive Rg3, Rg5, Rk1, and CK from Fermented Black Ginseng Using Novel Aspergillus niger KHNT-1 Strain Isolated from Korean Traditional Food. Processes 2021, 9, 227. [Google Scholar] [CrossRef]
- Quan, L.-H.; Piao, J.-Y.; Min, J.-W.; Yang, D.-U.; Lee, H.N.; Yang, D.C. Bioconversion of ginsenoside Rb1 into compound K by Leuconostoc citreum LH1 isolated from kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, T.; Hu, C.; Li, W.; Meng, Y.; Li, H.; Song, C.; He, C.; Zhou, Y.; Fan, Y. Ginsenoside compound K protects against obesity through pharmacological targeting of glucocorticoid receptor to activate lipophagy and lipid metabolism. Pharmaceutics 2022, 14, 1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, J.; Xu, J.; Qiu, Z.; Yao, F. Ginsenoside CK inhibits obese insulin resistance by activating PPARγ to interfere with macrophage activation. Microb. Pathog. 2021, 157, 105002. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Chun, S. Ginsenoside CK Inhibits the Early Stage of Adipogenesis via the AMPK, MAPK, and AKT Signaling Pathways. Antioxidants 2022, 11, 1890. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.-J.; Zhao, L.-C.; Zheng, Y.-N.; Chen, L.; Yang, G.-L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, D.; Li, J.; Yuan, G.; Li, H.; Xu, G.; Han, X.; Du, P.; An, L. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia 2014, 95, 58–64. [Google Scholar] [CrossRef]
- Yoon, S.H.; Han, E.J.; Sung, J.H.; Chung, S.H. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol. Pharm. Bull. 2007, 30, 2196–2200. [Google Scholar] [CrossRef]
- Song, W.; Wei, L.; Du, Y.; Wang, Y.; Jiang, S. Protective effect of ginsenoside metabolite compound K against diabetic nephropathy by inhibiting NLRP3 inflammasome activation and NF-κB/p38 signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Int. Immunopharmacol. 2018, 63, 227–238. [Google Scholar] [CrossRef]
- Cho, W.; Oh, H.; Abd El-Aty, A.; Hacimuftuoglu, A.; Jeong, J.H.; Jung, T.W. Therapeutic potential of ginsenoside compound K in managing tenocyte apoptosis and extracellular matrix damage in diabetic tendinopathy. Tissue Cell 2024, 86, 102275. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, Z.; Wu, S.; Chen, C.; Liu, Y.; Wang, M.; Zhang, Y.; Li, L.; Zou, H.; Zhao, G. Ginsenoside compound-K attenuates OVX-induced osteoporosis via the suppression of RANKL-induced osteoclastogenesis and oxidative stress. Nat. Prod. Bioprospect. 2023, 13, 49. [Google Scholar] [CrossRef]
- Ding, L.; Gu, S.; Zhou, B.; Wang, M.; Zhang, Y.; Wu, S.; Zou, H.; Zhao, G.; Gao, Z.; Xu, L. Ginsenoside compound K enhances fracture healing via promoting osteogenesis and angiogenesis. Front. Pharmacol. 2022, 13, 855393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Liu, W.-J.; Wen, M.-L.; Liang, H.; Wu, S.-M.; Zhu, Y.-Z.; Zhao, J.-Y.; Dong, X.-Q.; Li, M.-G.; Bian, L.; et al. Ameliorative effects of Compound K and ginsenoside Rh1 on non-alcoholic fatty liver disease in rats. Sci. Rep. 2017, 7, 41144. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.T.; Iseli, T.J.; Hoy, A.J.; George, J.; Grewal, T.; Roufogalis, B.D. Compound K modulates fatty acid-induced lipid droplet formation and expression of proteins involved in lipid metabolism in hepatocytes. Liver Int. 2013, 33, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Han, H.; Shi, H.; Wang, T.; Wang, B.; Zhao, J. Compound K, a metabolite of ginseng saponin, induces apoptosis of hepatocellular carcinoma cells through the mitochondria-mediated caspase-dependent pathway. Int. J. Clin. Exp. Med. 2017, 10, 11146–11156. [Google Scholar]
- Awais, M.; Akter, R.; Boopathi, V.; Ahn, J.C.; Lee, J.H.; Mathiyalagan, R.; Kwak, G.-Y.; Rauf, M.; Yang, D.C.; Lee, G.S. Discrimination of Dendropanax morbifera via HPLC fingerprinting and SNP analysis and its impact on obesity by modulating adipogenesis-and thermogenesis-related genes. Front. Nutr. 2023, 10, 1168095. [Google Scholar] [CrossRef]
- Lamichhane, G.; Pandeya, P.R.; Lamichhane, R.; Rhee, S.-j.; Devkota, H.P.; Jung, H.-J. Anti-obesity potential of ponciri fructus: Effects of extracts, fractions and compounds on adipogenesis in 3T3-L1 preadipocytes. Molecules 2022, 27, 676. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Peng, D. The exchangeable apolipoproteins in lipid metabolism and obesity. Clin. Chim. Acta 2020, 503, 128–135. [Google Scholar] [CrossRef]
- De Sereday, M.S.; Gonzalez, C.; Giorgini, D.; De Loredo, L.; Braguinsky, J.; Cobeñas, C.; Libman, C.; Tesone, C. Prevalence of diabetes, obesity, hypertension and hyperlipidemia in the central area of Argentina. Diabetes Metab. 2004, 30, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yang, Y.; Zhou, Y.; Wei, H.; Peng, J. GPR120: A critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell. Mol. Life Sci. 2017, 74, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Taherkhani, S.; Suzuki, K.; Ruhee, R.T. A brief overview of oxidative stress in adipose tissue with a therapeutic approach to taking antioxidant supplements. Antioxidants 2021, 10, 594. [Google Scholar] [CrossRef]
- Shahzad, N.; Alzahrani, A.R.; Ibrahim, I.A.A.; Shahid, I.; Alanazi, I.M.; Falemban, A.H.; Imam, M.T.; Mohsin, N.; Azlina, M.F.N.; Arulselvan, P. Therapeutic strategy of biological macromolecules based natural bioactive compounds of diabetes mellitus and future perspectives: A systemic review. Heliyon 2024, 10, e24207. [Google Scholar] [CrossRef]
- Wei, S.; Li, W.; Yu, Y.; Yao, F.; Lixiang, A.; Lan, X.; Guan, F.; Zhang, M.; Chen, L. Ginsenoside Compound K suppresses the hepatic gluconeogenesis via activating adenosine-5′ monophosphate kinase: A study in vitro and in vivo. Life Sci. 2015, 139, 8–15. [Google Scholar] [CrossRef]
- Riley, G. Chronic tendon pathology: Molecular basis and therapeutic implications. Expert Rev. Mol. Med. 2005, 7, 1–25. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover—Role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Pavone, V.; Testa, G.; Giardina, S.M.C.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological Therapy of Osteoporosis: A Systematic Current Review of Literature. Front. Pharmacol. 2017, 8, 803. [Google Scholar] [CrossRef]
- Tang, M.; Xie, X.; Yang, Y.; Li, F. Ginsenoside compound K—A potential drug for rheumatoid arthritis. Pharmacol. Res. 2021, 166, 105498. [Google Scholar] [CrossRef]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, G.; Chen, M.; Li, Y.; Tang, X.; Dai, Y. Therapeutic potential of alkaloid extract from Codonopsis Radix in alleviating hepatic lipid accumulation: Insights into mitochondrial energy metabolism and endoplasmic reticulum stress regulation in NAFLD mice. Chin. J. Nat. Med. 2023, 21, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.-T.; Su, H.-Y.; An, W. Glycosyltransferases and non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 2483. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Kumar, S.; Wong, R.; Newberry, C.; Yeung, M.; Peña, J.M.; Sharaiha, R.Z. Multidisciplinary Clinic Models: A Paradigm of Care for Management of NAFLD. Hepatology 2021, 74, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Y.; Wu, L.; Peng, J. Natural products in non-alcoholic fatty liver disease (NAFLD): Novel lead discovery for drug development. Pharmacol. Res. 2023, 196, 106925. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Li, D.; Fan, S.; Tao, F.; Yu, Y.; Lu, W.; Chen, Q.; Yuan, A.; Wu, J.; Zhao, G. Long-term and liver-selected ginsenoside C–K nanoparticles retard NAFLD progression by restoring lipid homeostasis. Biomaterials 2023, 301, 122291. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Zeng, X.; Liao, J.; Ouyang, D. Ginsenoside compound K alleviates sodium valproate-induced hepatotoxicity in rats via antioxidant effect, regulation of peroxisome pathway and iron homeostasis. Toxicol. Appl. Pharmacol. 2020, 386, 114829. [Google Scholar] [CrossRef]
- Ren, S.; Liu, R.; Wang, Y.; Ding, N.; Li, Y. Synthesis and biological evaluation of Ginsenoside Compound K analogues as a novel class of anti-asthmatic agents. Bioorg. Med. Chem. Lett. 2019, 29, 51–55. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Zhang, Y.; Li, J.; Wang, C.; Zhou, L.; Jia, Y.; Li, X. Synthesis and Biological Evaluation of Ginsenoside Compound K Derivatives as a Novel Class of LXRα Activator. Molecules 2017, 22, 1232. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Zhao, X.; Wang, Z.; Li, W.; Li, X.; Zheng, Y. Octyl ester of ginsenoside compound K as novel anti-hepatoma compound: Synthesis and evaluation on murine H22 cells in vitro and in vivo. Chem. Biol. Drug Des. 2018, 91, 951–956. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, X.-M.; Hu, J.-N.; Ye, H.; Luo, T.; Liu, X.-R.; Li, H.-Y.; Li, W.; Zheng, Y.-N.; Deng, Z.-Y. Absorption mechanism of ginsenoside compound K and its butyl and octyl ester prodrugs in Caco-2 cells. J. Agric. Food Chem. 2012, 60, 10278–10284. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-K.; Yan, X.-M.; Li, Z.-N.; Yan, Q.; Gong, X.-J. Synthesis and antitumor activity of three novel ginsenoside M1 derivatives with 3′-ester modifications. Bioorg. Chem. 2019, 90, 103061. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xin, J.; Zhang, Z.; Yan, H.; Wang, J.; Sun, E.; Hou, J.; Jia, X.; Lv, H. TPGS-modified liposomes for the delivery of ginsenoside compound K against non-small cell lung cancer: Formulation design and its evaluation in vitro and in vivo. J. Pharm. Pharmacol. 2016, 68, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Farh, M.E.-A.; Yang, D.C. Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: In vitro studies. J. Nanopart. Res. 2017, 19, 257. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Li, Y.; Li, W.; Zhou, J.; Chen, J.; Shang, Z.; Gu, Q.; Wang, W.; Shen, T.; et al. Micelles modified with a chitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells. Carbohydr. Polym. 2020, 230, 115576. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, M.; Hao, X.; Li, C.; Gao, Y.; Tao, J. Acute toxicity of sodium formononetin-3′-sulphonate (Sul-F) in Sprague-Dawley rats and Beagle dogs. Regul. Toxicol. Pharmacol. 2015, 73, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, M.; Mathiyalagan, R.; Boopathi, V.; Kong, B.M.; Choi, S.-K.; Lee, C.-S.; Yang, D.C.; Kang, S.C.; Thambi, T. Production of Minor Ginsenoside CK from Major Ginsenosides by Biotransformation and Its Advances in Targeted Delivery to Tumor Tissues Using Nanoformulations. Nanomaterials 2022, 12, 3427. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Z.K.; Li, C.Y.; Liang, Y.Q.; Yang, F. Anticancer properties and pharmaceutical applications of ginsenoside compound K: A review. Chem. Biol. Drug Des. 2022, 99, 286–300. [Google Scholar] [CrossRef] [PubMed]





| Name | Compound K |
|---|---|
| Alias | 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol |
| CAS number | 39262-14-1 |
| Pubchem CID | 9852086 |
| Compound type | tetra-cyclic tri-terpenoid |
| Molecular formula | C36H62O8 |
| Molecular weight | 622.9 g/mol |
| Form | powder |
| color | White |
| Solubility | DMF: 10 mg/mL; DMSO: 10 mg/mL; DMSO: PBS (pH 7.2) (1:1): 0.5 mg/mL |
| Density | 1.19 |
| pka | 12.94 ± 0.70 (Predicted) |
| Melting point | 181~183 °C |
| Boiling point | 723.1 ± 60.0 °C |
| LogP | 5.500 |
| Stability | Hygroscopic |
| Physicochemical Properties | Compound K | Standard Range |
|---|---|---|
| Molecular weight (g/mol) | 622.9 | <500 |
| Num. rotatable bonds | 7 | |
| Num. H-bond acceptors | 8 | ≤10 |
| Hydrogen bond donor | 6 | ≤5 |
| Molar Refractivity | 172.26 | 40–130 |
| TPSA (Å2) | 139.84 | <140 Å2 |
| Lipinski | Yes; 2 violations | |
| Bioavailability Score | 0.17 | >0.1 |
| ADME | ||
| Human Intestinal Absorption | 54.344 | |
| GI absorption | Low | |
| Blood–Brain Barrier Permeability | −1.038 | 0–1 |
| Volume distribution | 1.061 | 0.04–20 L/Kg |
| Plasma–protein binding | 93.57% | <90% |
| Total Clearance (log ml/min/kg) | 0.46 | |
| CYP1A2 inhibitor | No | 0–1 |
| CYP2C19 inhibitor | No | 0–1 |
| CYP2C9 inhibitor | No | 0–1 |
| CYP2D6 inhibitor | No | 0–1 |
| CYP3A4 inhibitor | No | 0–1 |
| Toxicity | ||
| Hepatotoxicity | Active (0.69) | 0–1 |
| Carcinogenesis | Inactive (0.62) | 0–1 |
| Immunotoxicity | Active (0.96) | 0–1 |
| Mutagenicity | Inactive (0.97) | 0–1 |
| Cytotoxicity | Inactive (0.93) | 0–1 |
| Mitochondrial Membrane Potential | Inactive (0.70) | 0–1 |
| Disease | Experimental Models | Dosage Form | Doses of Administrations | Mechanism | Ref. |
|---|---|---|---|---|---|
| Obesity | C57BL/6J mice | Oral | 15, 30, 60 mg/kg |
| [13] |
| Male C57BL/6J and ob/ob (B6/JGpt-Lepem1Cd25/Gpt) mice | i.p. injection | 20 mg/kg |
| [77] | |
| 3T3-L1 cell lines | Cell treatment | 0.05, 0.5, 5 μM |
| [19] | |
| 3T3-L1 cell lines | Cell treatment | 20, 50 μM |
| [78] | |
| 3T3-L1 cell lines | Cell treatment | 10–40 μM |
| [79] | |
| Diabetes | male ICR mice | Oral | 30 mg/kg/day |
| [80] |
| Male Wistar rats (200–250 g) | Oral | 30, 100, 300 mg/kg BW |
| [81] | |
| MIN6 cell line | Cell treatment | 2–32 μM |
| [34] | |
| male C57BL/KsJ db/db mice | Oral | CK: Metformin 1:15 |
| [82] | |
| DN | HFD (high-fat diet)/STZ (streptozotocin)-induced DN mice model | Intragastrically | 10, 20, 40 mg/kg/day |
| [83] |
| DT | human tenocytes cell | Cell treatment | 3, 10 μM |
| [84] |
| OP |
Raw264.7 cells Balb/C female mice |
Cell treatment, i.p. injection | 10 μM 10 mg/kg |
| [85] |
|
bone marrow mesenchymal stem cells male Sprague Dawley (SD) rats |
Cell treatment, i.p. injection | 2.5–40 μM 10 μM |
| [86] | |
| OA | MC3T3E1 cell lines | Cell treatment | 0.01–10 μM |
| [8] |
| NAFLD |
SD rats, HSC-T6 cells |
i.p. injection Cell treatment | 3 mg/kg/day |
| [87] |
| HepG2 cells | Cell treatment | 20 μM |
| [30] | |
| HuH7 cells | Cell treatment | 1 μM |
| [88] | |
| HCC | HepG2 cells | Cell treatment | 0, 5, 10 μmol |
| [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morshed, M.N.; Akter, R.; Karim, M.R.; Iqbal, S.; Kang, S.C.; Yang, D.C. Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases. Curr. Issues Mol. Biol. 2024, 46, 2320-2342. https://doi.org/10.3390/cimb46030148
Morshed MN, Akter R, Karim MR, Iqbal S, Kang SC, Yang DC. Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases. Current Issues in Molecular Biology. 2024; 46(3):2320-2342. https://doi.org/10.3390/cimb46030148
Chicago/Turabian StyleMorshed, Md. Niaj, Reshmi Akter, Md. Rezaul Karim, Safia Iqbal, Se Chan Kang, and Deok Chun Yang. 2024. "Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases" Current Issues in Molecular Biology 46, no. 3: 2320-2342. https://doi.org/10.3390/cimb46030148
APA StyleMorshed, M. N., Akter, R., Karim, M. R., Iqbal, S., Kang, S. C., & Yang, D. C. (2024). Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases. Current Issues in Molecular Biology, 46(3), 2320-2342. https://doi.org/10.3390/cimb46030148