1. Introduction
Cognitive impairment refers to a decline in cognitive function that affects various cognitive domains and can impact daily functioning. Severe cognitive impairment is often considered equivalent to dementia, which is characterized by chronic cognitive decline in multiple domains leading to a significant decline in daily activities [
1]. Dementia is not solely a normal part of aging but rather a condition that significantly affects individuals’ quality of life and independence. It is essential to distinguish between mild cognitive impairment, where cognitive function is impaired to a greater extent than is expected to be due to age, but the patient does not meet the criteria for dementia [
2].
To diagnose cognitive impairment, especially in its early stages, a combination of clinical assessments and biomarkers plays a crucial role. Clinical examinations, including cognitive tests such as Montreal Cognitive Assessment (MoCA) and assessments of daily functioning, are typically used as the primary method for diagnosing cognitive impairment [
3]. However, biomarkers can significantly aid in the early diagnosis of cognitive impairment, particularly in individuals without evident clinical signs of neurocognitive decline [
4].
In the study by Battaglia et al. (2024), the focus was on the neural correlates and molecular mechanisms underlying memory and learning. The research emphasizes the critical role of memory and learning in acquiring, storing, and retrieving information essential for cognitive processes. This understanding is particularly relevant in the context of cognitive decline, where disruptions in memory and learning processes are often early indicators. By exploring the neural underpinnings and molecular pathways involved in memory and learning, this study contributes to a deeper understanding of how these cognitive functions are orchestrated at the neural level and how their impairment can lead to cognitive decline and neurodegenerative diseases such as Alzheimer’s [
5].
MRI is a valuable tool in diagnosing cognitive impairment, particularly in conditions such as mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Brodmann areas are distinct regions of the cerebral cortex identified based on cytoarchitectural variance. Mapping these areas using MRI can offer crucial insights into cognitive function and dysfunction [
6].
The study by Battaglia et al. (2024) focuses on utilizing a multiscale integrated approach to investigate functional connectivity in fear learning. The research emphasizes the significance of functional brain connectivity assessments, particularly through techniques such as functional magnetic resonance imaging (fMRI). By employing fMRI, the study aims to gain valuable insights into how different brain regions interact and communicate during fear learning processes. Functional magnetic resonance imaging plays a crucial role in elucidating the neural mechanisms involved in fear learning, providing a deeper understanding of the functional connectivity patterns within the brain during these cognitive processes and could possibly aid in early diagnosis of cognitive decline [
7].
Research has shown that MRI activation maps can be overlaid on automated anatomical labeling templates to estimate activation in Brodmann areas, aiding in visualizing cognitive processes and dysfunction [
8]. Specifically, studies have indicated selective atrophy in Brodmann area 35 (BA35), approximating the trans-entorhinal region, in individuals with preclinical AD compared to controls, highlighting the utility of MRI in detecting early structural changes linked to cognitive decline [
9]. Furthermore, alterations in cortical thickness can be indicative of cognitive decline, with specific regions such as the entorhinal cortex and precuneus playing crucial roles in cognitive impairment [
10,
11].
Alterations in cortical thickness can serve as indicators of cognitive decline due to the close relationship between brain structure and cognitive function. Changes in cortical thickness have been associated with various cognitive processes and disorders. For instance, studies have shown that alterations in cortical thickness in regions such as Brodmann Area 44, involved in language processing, and the primary motor cortex (BA4Ap) have shown changes in cortical thickness that may affect cognitive abilities [
12,
13]. Moreover, cortical thickness alterations in areas such as the entorhinal cortex (BA28) have been linked to memory deficits and cognitive decline, particularly in conditions such as Alzheimer’s disease [
14]. Changes in cortical thickness in language-related regions, including Broca’s area (BA44) and peri-sylvian language networks, have been associated with language impairment and cognitive dysfunction [
15,
16]. Furthermore, structural connectivity deficits in regions such as the inferior frontal gyrus (BA44) have been observed in individuals with speech disorders, highlighting the role of cortical thickness in language function and cognitive processes [
17].
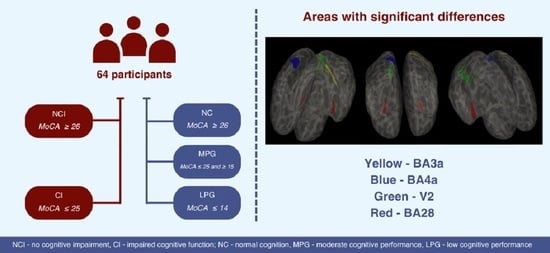
This work aims to compare cortical thickness measurements in different areas of the brain divided using Brodmann area map parcellation in two ways: between the two groups—patients without cognitive impairment (NCI) and patients with impaired cognitive function (CI) according to MoCA, and also between the three groups (normal cognition (NC), moderate cognitive performance group (MPG) and low cognitive performance group (LPG)), which were divided based on the results of the MoCA test.
2. Materials and Methods
A total of 64 participants were enrolled in the cross-sectional study and then were divided into groups in two ways according to the Montreal Cognitive Assessment (MoCA) score:
In two groups (with a score ≥ 26 and with a score <25);
In three groups (with a score ≥ 26, with a score ≥ 15 and ≤25 and with a score ≤ 14).
Division in two groups. Patients without cognitive impairment (NCI) according to MoCA (with a score ≥ 26) and patients with impaired cognitive function (CI) according to MoCA (with a score < 25) [
18].
Research participant demographic data, gender, and MoCA scores between the two groups can be seen in
Table 1.
A Chi-square test on gender was performed, determining that there was a statistically significant difference between the groups (X2 = 4.512, p = 0.034). A Mann–Whitney U test did not find age differences in groups (U = 363.000, p = 0.584). MoCA scores were significantly different between the two groups (U = 799.000, p < 0.001).
Division in three groups. To better assess differences between severe cognitive impairment and mild/moderate cognitive impairment patients were divided as follows:
Normal cognition group (NC)—participants with MoCA scores ≥ 26;
Moderate cognitive performance group (MPG)—participants with MoCA ≥ 15 and ≤25;
Low cognitive performance group (LPG)—participants with MoCA ≤ 14.
There were 17 participants in the NC group (mean age 70.941, SD 7.554, youngest 51 year, oldest 83 years. Mean MoCA score 27.529 (SD 1.231, lowest score 26, highest score 30).
MPG group contained 33 patients (mean age 72.758, SD 7.293, youngest 57 years, oldest 85 years). Mean MoCA score 22.121 (SD 2.792, lowest score 15, highest score 25).
LPG group contained 14 patients (mean age 74.286, SD 10.373, youngest 62, oldest 96). Mean MoCA score 8.786 (SD 3.786, lowest score 4, highest score 14). Research participant demographic data, gender, and MoCA scores between the three groups can be seen in
Table 2.
A Chi-square test on gender was performed, determining that there were no statistically significant differences between the groups (X2 = 4.631, p = 0.099). Similarly, Kruskall–Wallis tests did not find age differences in groups (H (2) = 0.340, p = 0.844). MoCA scores were significantly different between the three groups (H (2) = 52.795, p < 0.001).
2.1. Selection of Participants
In our study, individuals were referred to a board-certified neurologist specializing in cognitive impairment for evaluation due to either subjective cognitive complaints or suspected cognitive decline. All of the selected patients had right hand dominance. Exclusion criteria encompassed clinically significant neurological conditions such as tumors, major strokes, intracerebral lobar hemorrhages, malformations, Parkinson’s disease, and multiple sclerosis, as well as substance, alcohol abuse, major depression, schizophrenia, and other psychiatric conditions. Additionally, patients with documented significant vascular diseases were not considered for inclusion. Magnetic resonance imaging (MRI) did not reveal any other noteworthy pathological findings in the study participants.
2.2. MRI Acquisition Protocol
MRI imaging was conducted using a 3.0 tesla MRI scanner within the setting of a university hospital. 3D T1 Ax (flip angle 11, TE min full, TI 400, FOV 25.6, layer thickness 1 mm) images were used for post-processing. Additional sequences were used to rule out other clinically significant pathologies, including, T2, 3D FLAIR, DWI, ADC, SWI sequences.
2.3. Cortical Parcellation
Cortical reconstruction was performed by using Freesurfer 7.2.0 image analysis software. It is documented and freely available for download online (
http://surfer.nmr.mgh.harvard.edu/ accessed on 16 February 2024). The technical details of these procedures are described in prior publications [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34].
We used the Brodmann Area Maps (BA Maps) and Hinds V1 Atlas labelling protocol to extract cortical thickness results [
35,
36,
37,
38,
39,
40].
2.4. Statistical Analysis
JASP 0.18.3 was used for the statistical analysis (Eric-Jan Wagenmakers, Amsterdam, The Netherlands) [
41]. Statistical analysis included descriptive statistics, a Chi-square test, a Mann–Whitney U test, a Kruskal–Wallis test, and Dunn’s post hoc analysis of study results.
Descriptive statistics were used to estimate general variables and differences between groups. A Chi-square test was used to determine the association between categorical variables. A Mann–Whitney U test was used to evaluate statistically significant differences between the patients without cognitive impairment and patients with impaired cognitive function according to MoCA. A Kruskall–Wallis test was used to evaluate statistically significant differences between the NC, MPG and LPG groups, and if there were statistically significant differences, Dunn’s post hoc test was utilized with additional Bonferroni and Holm corrections.
4. Discussion
Till today scientists all around the world are trying to find early markers of dementia and cognitive impairment. Cortical thickness measurements have emerged as a valuable biomarker for cognitive impairment in various neurological conditions. In our study we tried to find connections between cortical thickness of different brain regions according to Brodmann area map and cognitive impairment. In discussion we compare our results with other research papers and provide possible explanations for our findings, as well as future directions.
BA4a is a primary anterior motor area responsible for motor planning and execution. Changes in cortical thickness in BA4a may influence motor control and coordination, which can impact cognitive functions related to motor skills and coordination [
42,
43]. Our study showed statistically significant results in NCI and CI groups in BA4a in the right hemisphere. One study suggests that cortical thickness alterations in BA4a and other motor areas may be associated with motor deficits and cognitive impairment in individuals with Parkinson’s disease [
44].
BA3a is a primary somatosensory area involved in processing sensory information. Alterations in cortical thickness in BA3a may affect sensory perception, potentially impacting cognitive functions related to sensory processing and discrimination [
45,
46]. Our study showed statistically significant results between LPG, MPG, and NC groups and in the NCI and CI groups in BA3a in the left hemisphere. The results of one study suggest that alterations in cortical thickness in BA3b, a primary somatosensory area, may impact texture discrimination and perception, potentially contributing to cognitive impairment in individuals with these deficits [
47].
V2 is the secondary visual cortex essential for visual processing and perception. It is involved in higher-order visual processing tasks such as color perception, motion detection, and object recognition. The connection between V2 and cognitive functioning is significant as V2 contributes to the integration and interpretation of visual stimuli, which are essential for various cognitive processes. It receives the information from V1 and process the information to other parts of the cortex [
48]. Alterations in cortical thickness in V2 may affect visual perception and recognition, potentially impacting cognitive functions related to visual information processing and object recognition [
49]. Our study showed statistically significant results between LPG, MPG, and NC groups in V2 in the right hemisphere. Additionally, it showed nearly significant results in the NCI and CI groups. Previously association between cortical thickness alterations in V2 region and cognitive impairment was observed individuals with Huntington’s disease [
50].
The most promising region in a role of a biomarker proved to be entorhinal cortex (BA28) in both hemispheres, since it showed statistically significant results between LPG, MPG, and NC groups on both sides. Additionally, entorhinal cortex in the left hemisphere showed statistically significant results in the NCI and CI groups and in the right hemisphere showed nearly significant result between two groups. The entorhinal cortex (BA28) in both the left and right hemispheres plays a crucial role in spatial memory and episodic memory functions [
51]. It is involved in navigation, formation, and consolidation of spatial and declarative memory [
52]. The left entorhinal cortex is associated with object recognition and familiarity-based judgments [
53]. Changes in the right entorhinal cortex have been associated with memory deficits and cognitive decline, highlighting its importance in cognitive function [
54]. Additionally, the entorhinal cortex is correlated with episodic memory and is impacted by Alzheimer’s disease-related pathology [
55]. The results reinforce studies that linked entorhinal cortex thickness to cognitive impairment [
56,
57,
58].
Similar studies collectively emphasize the importance of investigating cognitive functions, brain volumes, metacognitive abilities, and white matter integrity in individuals with cognitive impairment, particularly in the context of mathematical knowledge, financial capacity, and related cognitive functions in conditions such as amnestic mild cognitive impairment (aMCI) and Alzheimer’s disease.
The study by Giannouli and Tsolaki (2023) focused on investigating brain volumes and metacognitive deficits in individuals with amnestic Mild Cognitive Impairment (aMCI) compared to healthy controls. They found positive correlations between knowledge of strategies (avoidance strategies) and amygdala on both sides and white matter volume [
59]. In a study by Stoeckel et al. (2013), the researchers explored the relationship between the MRI volume of the medial frontal cortex and financial capacity in individuals with mild Alzheimer’s disease. The study indicated that the volume of the medial frontal cortex could predict financial capacity in patients with mild Alzheimer’s disease [
60]. Giannouli and Tsolaki (2019) found a strong correlation between financial capacity in aMCI patients and the volumes of the right amygdala and left angular gyrus [
61]. Gerstenecker et al. (2017) conducted a study focusing on the association between white matter degradation and reduced financial capacity in individuals with mild cognitive impairment (MCI) and Alzheimer’s disease (AD). The research highlighted that in AD, increased mean, and axial diffusivities in specific brain regions such as the anterior cingulate, callosum, and frontal areas were linked to poorer financial capacity [
62].
By analyzing our data, we did not find significant differences in the NCI and CI groups, and similarly in the LPG, MPG, and NC groups apart from the structures mentioned above. This study was exploratory rather than confirmatory and performed on a small cohort. When presenting the p value, multiple comparison corrections were not made. Therefore, the results serve as exploratory data to be validated by a larger cohort and further multiple comparison corrections.
One of the primary limitations of this study is the relatively small sample size of 64 patients. A larger sample would provide more robust and generalizable findings. Another possible limitation is the cross-sectional design of the study. Longitudinal studies would offer more insights into the progression and predictive value of cortical thickness changes over time. Also, depression is known to impact cognitive functions and could potentially confound the relationship between cortical thickness measurements and cognitive impairment. The study does not account for the potential effects of depression cognitive-neuropsychological, and emotional characteristics of the participants on cognitive performance and cortical thickness. In the end, while some differences in cortical thickness reached statistical significance, the clinical significance of these findings in terms of actual cognitive impairment and functional outcomes remains to be determined.
Future studies should integrate multimodal imaging techniques such as functional MRI (fMRI) and diffusion tensor imaging (DTI) to examine the functional and structural connectivity changes related to cortical thickness alterations. Additionally, it would be beneficial to compare cortical thickness measurements from BA mapping with other cognitive assessment tools to assess the reliability and consistency of the results. Furthermore, research should focus on investigating the specific roles of the identified Brodmann areas (BA3a, BA4a, BA28, V2) in cognitive processes and include comprehensive cognitive-neuropsychological and emotional assessments to understand their influence on cortical thickness measurements and cognitive performance.
Utilizing cortical thickness measurements as biomarkers can aid in early diagnosis and intervention for cognitive impairment, improving the accuracy of diagnosis and allowing for personalized treatment plans. Additionally, identifying specific Brodmann areas linked to cognitive impairment can guide the development of educational and counseling programs to enhance cognitive function and quality of life for those experiencing cognitive decline.