Structural Characterization and Anti-HSV-1 and HSV-2 Activity of Glycolipids from the Marine Algae Osmundaria obtusiloba Isolated from Southeastern Brazilian Coast
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipid Fractionation

2.2. Mass Spectrometry of Neutral Glycolipids

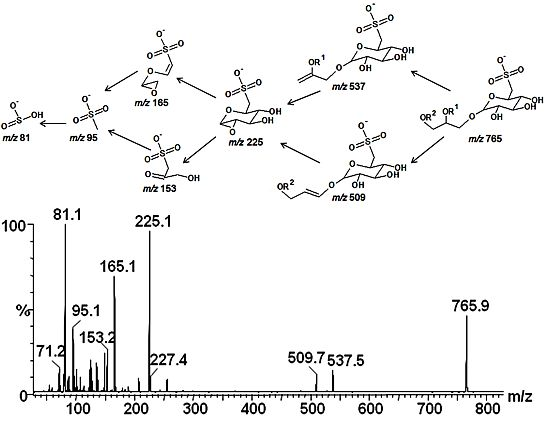
2.3. Mass Spectrometry of Sulfolipids


2.4. NMR Spectroscopy of Sulfolipids

3. Experimental Section
3.1. Biological Material
3.2. Extraction and Fractionation of Lipids
3.3. Mass Spectrometry
3.4. Nuclear Magnetic Resonance
3.5. Cells and Viruses
3.6. Cytotoxicity Assay
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry 2004, 65, 721–730. [Google Scholar]
- Kim, Y.H.; Kim, E.H.; Lee, C.; Kim, M.H.; Rho, J.R. Two new monogalactosyl diacylglycerols from brown alga Sargassum thunbergii. Lipids 2007, 42, 395–399. [Google Scholar]
- Illijas, M.I.; Indy, J.R.; Yasui, H.; Itabashi, Y. Lipid class and fatty acid composition of a little-known and rarely collected alga Exophyllum wentii Weber-van Bosse from Bali Island, Indonesia. J. Oleo Sci. 2009, 58, 103–110. [Google Scholar]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata (Kutz.) Okamura. Glycobiology 2006, 16, 902–915. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Gorin, P.A.J.; Tischer, C.A.; Iacomini, M. Sulfonoglycolipids from the lichenized basidiomycete Dictyonema glabratum: Isolation, NMR, and ESI-MS approaches. Glycobiology 2001, 11, 345–351. [Google Scholar]
- Souza, L.M.; Iacomini, M.; Gorin, P.A.J.; Sari, R.S.; Haddad, M.A.; Sassaki, G.L. Glyco- and sphingophosphonolipids from the medusa Phyllorhiza punctata: NMR and ESI-MS/MS fingerprints. Chem. Phys. Lipids 2007, 145, 85–96. [Google Scholar]
- Gustafson, K.R.; Cardellina, J.H.; Fuller, R.W.; Weislow, O.W.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.L.; Boyd, M.R. AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Nat. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar]
- Morimoto, T.; Nagatsu, A.; Murakami, N.; Sakakibara, J.; Tokuda, H.; Nishimo, H.; Iwashima, A. Antitumor promoting glyceroglycolipids from the green alga Chlorella vulgaris. Phytochemistry 1995, 40, 1433–1437. [Google Scholar]
- Ohta, K.; Mizushina, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of eukaryotic DNA polymerases and HIV-reverse transcriptase type 1 from a marine red alga, Gigartina tenella. Chem. Pharm. Bull. (Tokyo) 1998, 46, 684–686. [Google Scholar] [CrossRef]
- Naumann, I.; Darsow, K.H.; Walter, C.; Lange, H.A.; Buchholz, R. Identification of sulfoglycolipids from the alga Porphyridium purpureum by matrix-assisted laser desorption/ionisation quadrupole ion trap time of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3185–3192. [Google Scholar]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S.; Coto, C.E. Herpes simplex virus-inhibitory sulfated xylogalactans from the red seaweed Nothogenia fastigiata. Exp. Chemoter. 1996, 42, 57–64. [Google Scholar]
- Witvrouw, M.; de Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharm. 1997, 29, 497–511. [Google Scholar]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Res. 1999, 43, 93–102. [Google Scholar]
- Duarte, M.E.R.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef]
- Romanos, M.T.V.; Andrada-Serpa, M.J.; Mourão, P.A.S.; Yoneshigue-Valentin, Y.; Costa, S.S.; Pereira, M.S.; Miranda, M.M.F.S.; Gonçalves, J.L.S.; Wigg, M.D. A sulphated fucan from the Laminaria abyssalis inhibits the human T cell lymphotropic virus type 1-induced syncytium formation in HeLa cells. Antivir. Chem. Chemother. 2002, 13, 219–221. [Google Scholar]
- Duarte, M.E.R.; Cauduro, J.P.; Noseda, D.G.; Noseda, M.D.; Gonçalves, A.G.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S. The structure of the agaran sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and its antiviral activity. Relation between structure and antiviral activity in agarans. Carbohydr. Res. 2004, 339, 335–347. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.; Faria, P.C.; Noseda, M.D.; Duarte, M.E.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar]
- Wang, H.; Li, Y.L.; Shen, W.Z.; Rui, W.; Ma, X.J.; Cen, Y.Z. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV-1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Sci. Asia 2009, 35, 137–141. [Google Scholar]
- El-Baroty, G.S.; El-Baz, F.K.; Abd-Elmoein, A.; Abd El Baky, H.H.; Ali, M.M.; Ibrahim, A.E. Evaluation of glycolipids of some egyptian marine algae as a source of bioactive substances. EJEAFChe 2011, 10, 2114–2128. [Google Scholar]
- Mattos, B.B.; Romanos, M.T.V.; Souza, L.M.; Sassaki, G.L.; Barreto-Bergter, E. Glycolipids from macroalgae: potential biomolecules for marine biotechnology? Braz. J. Pharmacogn. 2011, 21, 244–247. [Google Scholar]
- Christophers, J.; Sutton, R.N. Characterisation of acyclovir-resistant and -sensitive clinical herpes simplex virus isolates from an immunocompromised patient. J. Antimicrob. Chemother. 1987, 20, 389–398. [Google Scholar]
- Barreto-Bergter, E.; Sassaki, G.L.; Souza, L.M. Structural analysis of fungal cerebrosides. Front. Microbiol. 2011, 2, 1–11. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Skipski, V.P. Thin layer chromatography of neutral glycolipids. Methods Enzymol. 1975, 35, 396–425. [Google Scholar]
- Kim, Y.H.; Gil, J.H.; Hong, J.; Yoo, J.S. Tandem mass spectrometric analysis of fatty acyl groups of galactolipid molecular species from wheat flour. Microchem. J. 2001, 68, 143–155. [Google Scholar]
- Scoparo, C.T.; Souza, L.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, I. Analysis of Camellia sinensis green and black teas via ultra high performance liquid chromatography assisted by liquid-liquid partition and two-dimensional liquid chromatography (size exclusion × reversed phase). J. Chromatogr. A 2012, 1222, 29–37. [Google Scholar]
- Souza, L.M.; Müller-Santos, M.; Iacomini, M.; Gorin, P.A.J.; Sassaki, G.L. Positive- and negative-tandem mass spectrometric fingerprints of lipids from the halophilic archaea Haloarcula marismortui. J. Lipid Res. 2009, 50, 1363–1373. [Google Scholar]
- Benson, A.A.; Daniel, H.; Wiser, R.A. A sulfolipid in plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1582–1587. [Google Scholar]
- Benson, A.A. The plant sulpholipid. Adv. Lipid Res. 1963, 1, 387–394. [Google Scholar]
- Naumann, I.; Klein, B.C.; Bartel, S.J.; Darsow, K.H.; Buchholz, R.; Lange, H.A. Identification of sulfoquinovosyldiacyglycerides from Phaeodactylum tricornutum by matrix-assisted laser desorption/ionization QTrap time-of-flight hybrid mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2517–2523. [Google Scholar]
- Zhang, X.; Fhaner, C.J.; Ferguson-Miller, S.M.; Reid, G.E. Evaluation of ion activation strategies and mechanisms for the gas-phase fragmentation of sulfoquinovosyldiacylglycerol lipids from Rhodobacter sphaeroides. Int. J. Mass Spectrom. 2012, in press.. [Google Scholar]
- Sassaki, G.L.; Machado, M.J.; Tischer, C.A.; Gorin, P.A.J.; Iacomini, M. Glycosyldiacylglycerolipids from the lichen Dictyonema glabratum. J. Nat. Prod. 1999, 62, 844–847. [Google Scholar]
- Siddantha, A.K.; Ramvat, B.K.; Chauvan, V.D.; Achari, B.; Dutta, P.K.; Pakrashi, S.C. Sulphoglycolipid from the green alga Enteromorpha flexuosa (Wulf). J. Agric. Bot. Mar. 1991, 34, 365–367. [Google Scholar]
- Siddhanta, A.K.; Mody, K.H.; Ramavat, B.K.; Chauan, V.D.; Sharma, M.; Garg, S.H. Characterization of Sulphonoglycolipid from the Red Alga Laurencia pedicularoides. Bot. Mar. 1995, 38, 329–331. [Google Scholar]
- Sassaki, G.L.; Riter, D.S.; Filho, A.S.P.; Guerrini, M.; Lima, M.A.; Cosentino, C.; Souza, L.M.; Cipriani, T.R.; Rudd, T.R.; Nader, H.B.; et al. A robust method to quantify low molecular weight contaminants in heparin: detection of tris(2-n-butoxyethyl) phosphate. Analyst 2011, 136, 2330–2338. [Google Scholar]
- Markoulatos, P.; Georgopoulou, A.; Siafakas, N.; Plakokefalos, E.; Tzanakaki, G.; Kourea-Kremastinou, J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J. Clin. Microbiol. 2001, 39, 4426–4432. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating 50 per cent end-points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Nishimura, T.; Toku, K.; Fukuyasu, H. Antiviral compounds. XII. Antiviral activity of aminohydrazones of alkoxyphenyl substituted carbonyl compounds against influenza virus in eggs and mice. Kitasato Arch. Exp. Med. 1977, 50, 39–46. [Google Scholar]
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Souza, L.M.; Sassaki, G.L.; Romanos, M.T.V.; Barreto-Bergter, E. Structural Characterization and Anti-HSV-1 and HSV-2 Activity of Glycolipids from the Marine Algae Osmundaria obtusiloba Isolated from Southeastern Brazilian Coast. Mar. Drugs 2012, 10, 918-931. https://doi.org/10.3390/md10040918
De Souza LM, Sassaki GL, Romanos MTV, Barreto-Bergter E. Structural Characterization and Anti-HSV-1 and HSV-2 Activity of Glycolipids from the Marine Algae Osmundaria obtusiloba Isolated from Southeastern Brazilian Coast. Marine Drugs. 2012; 10(4):918-931. https://doi.org/10.3390/md10040918
Chicago/Turabian StyleDe Souza, Lauro M., Guilherme L. Sassaki, Maria Teresa Villela Romanos, and Eliana Barreto-Bergter. 2012. "Structural Characterization and Anti-HSV-1 and HSV-2 Activity of Glycolipids from the Marine Algae Osmundaria obtusiloba Isolated from Southeastern Brazilian Coast" Marine Drugs 10, no. 4: 918-931. https://doi.org/10.3390/md10040918
APA StyleDe Souza, L. M., Sassaki, G. L., Romanos, M. T. V., & Barreto-Bergter, E. (2012). Structural Characterization and Anti-HSV-1 and HSV-2 Activity of Glycolipids from the Marine Algae Osmundaria obtusiloba Isolated from Southeastern Brazilian Coast. Marine Drugs, 10(4), 918-931. https://doi.org/10.3390/md10040918