Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis and Bioassay Guided Fractionation



2.2. Biological Activity of the D. orbita Compounds
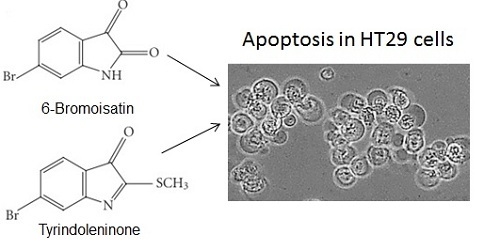
2.2.1. Apoptosis, Necrosis and Cell Viability



2.2.2. Cell Cycle Analysis

3. Experimental Section
3.1. Egg Mass Extraction, Purification
3.2. Chemical Analysis
3.3. Cell Culture
3.4. MTT Viability Assay and Cell Morphology
3.5. Combined Caspase 3/7, Membrane Integrity and Cell Viability Assays
3.6. Flow Cytometric Detection of Apoptosis
3.7. Cell Cycle Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Langley, K.; Exinger, F.; Gosse, F.; Raul, F. Geraniol, a component of plant essential oils, sensitizes human colonic cancer cells to 5-fluorouracil treatment. J. Pharmacol. Exp. Ther. 2002, 301, 625–630. [Google Scholar] [CrossRef]
- Line-Edwige, M. Antiproliferative effect of alcoholic extracts of some Gabonese medicinal plants on human colonic cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 112–117. [Google Scholar]
- Harvey, A.L. Natural products as a screening resource. Curr. Opin. Chem. Biol. 2007, 11, 480–484. [Google Scholar] [CrossRef]
- Harvey, A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Esmaeelian, B.; Kamrani, Y.Y.; Amoozegar, M.A.; Rahmani, S.; Rahimi, M.; Amanlou, M. Anti-cariogenic properties of malvidin-3,5-diglucoside isolated from Alcea longipedicellata against oral bacteria. Int. J. Pharmacol. 2007, 3, 468–474. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Agarwal, R. Natural products and colon cancer: Current status and future prospects. Drug Dev. Res. 2008, 69, 460–471. [Google Scholar] [CrossRef]
- Manson, M.M.; Farmer, P.B.; Gescher, A.; Steward, W.P. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005, 166, 257–275. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 30, 237–323. [Google Scholar]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar]
- Sato, M.; Sagawa, M.; Nakazato, T.; Ikeda, Y.; Kizaki, M. A natural peptide, dolastatin 15, induces G2/M cell cycle arrest and apoptosis of human multiple myeloma cells. Int. J. Oncol. 2007, 30, 1453–1459. [Google Scholar]
- Jiang, C.; Wang, M.; Liu, J.; Gan, D.; Zeng, X. Extraction, preliminary characterization, antioxidant and anticancer activities in vitro of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2011, 84, 851–857. [Google Scholar] [CrossRef]
- Baker, J. Tyrian purple: An ancient dye, a modern problem. Endeavour 1974, 33, 11–17. [Google Scholar] [CrossRef]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Tyrian purple precursors in the egg masses of the Australian muricid, Dicathais orbita: A possible defensive role. J. Chem. Ecol. 2000, 26, 1037–1050. [Google Scholar] [CrossRef]
- Cooksey, C.J. Tyrian purple: 6,6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–769. [Google Scholar] [CrossRef]
- Baker, J.T.; Duke, C.C. Isolation of choline and choline ester salts of tyrindoxyl sulphate from the marine mollusks Dicathais orbita and Mancinella keineri. Tetrahedron Lett. 1976, 1233–1234. [Google Scholar] [CrossRef]
- Westley, C.B.; Vine, K.L.; Benkendorff, K. A Proposed Functional Role for Indole Derivatives in Reproduction and Defense of the Muricidae (Neogastropoda: Mollusca). In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, L., Eisenbrand, G., Eds.; Life in Progress Editions: Roscoff, France, 2006; pp. 31–44. [Google Scholar]
- Westley, C.B.; McIver, C.M.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. Enhanced acute apoptotic response to azoxymethane-induced DNA damage in the rodent colonic epithelium by Tyrian purple precursors: A potential colorectal cancer chemopreventative. Cancer Biol. Ther. 2010, 9, 371–379. [Google Scholar] [CrossRef]
- Cooksey, C.J. Marine Indirubins. In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, L., Eisenbrand, G., Eds.; Life in Progress Editions: Roscoff, France, 2006; pp. 23–30. [Google Scholar]
- Westley, C.; Benkendorff, K. Sex-specific Tyrian purple genesis: Precursor and pigment distribution in the reproductive system of the marine mollusc, Dicathais orbita. J. Chem. Ecol. 2008, 34, 44–56. [Google Scholar] [CrossRef]
- Benkendorff, K. The Australian Muricidae Dicathais orbita: A model species for marine natural product research. Mar. Drugs 2013, 11, 1370–1398. [Google Scholar] [CrossRef]
- Benkendorff, K.; McIver, C.M.; Abbott, C.A. Bioactivity of the Murex homeopathic remedy and of extracts from an Australian muricid mollusc against human cancer cells. Evid. Based Complement. Altern. Med. 2011, 2011, 879585. [Google Scholar]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Benkendorff, K.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931–938. [Google Scholar] [CrossRef]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. J. Biol. Chem. 2001, 276, 251–260. [Google Scholar]
- Noble, W.J.; Cocks, R.R.; Harris, J.O.; Benkendorff, K. Application of anaesthetics for sex identification and bioactive compound recovery from wild Dicathais orbita. J. Exp. Mar. Biol. Ecol. 2009, 380, 53–60. [Google Scholar] [CrossRef]
- Benkendorff, K. Aquaculture and the Production of Pharmaceuticals and Nutraceuticals; Woodhead Publishing: Cambridge, UK, 2009; pp. 866–891. [Google Scholar]
- Westley, C.B.; Benkendorff, K.; McIver, C.M.; Le Leu, R.K.; Abbott, C.A. Gastrointestinal and hepatotoxicity assessment of an anticancer extract from muricid molluscs. Evid. Based Complement. Altern. Med. 2013, 2013, 837370. [Google Scholar]
- Baker, J.; Duke, C. Chemistry of the indoleninones. II. Isolation from the hypobranchial glands of marine molluscs of 6-Bromo-2,2-dimethylthioindolin-3-one and 6-Bromo-2-methylthioindoleninone as alternative precursors to Tyrian purple. Aust. J. Chem. 1973, 26, 2153–2157. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Review overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Vine, K.L. An Investigation into the Cytotoxic Properties of Isatin-Derived Compounds: Potential for Use in Targeted Cancer Therapy. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 14 September 2007. [Google Scholar]
- Rochat, B. Importance of influx and efflux systems and xenobiotic metabolizing enzymes in intratumoral disposition of anticancer agents. Curr. Cancer Drug Targets 2009, 9, 652–674. [Google Scholar] [CrossRef]
- Vine, K.; Matesic, L.; Locke, J.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000–2008. Anticancer Agents Med. Chem. 2009, 9, 397–414. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.-A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Nguyen, J.T.; Wells, J.A. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7533–7538. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2004, 2, 51–62. [Google Scholar] [CrossRef]
- Pozhilenkova, E.; Salmina, A.; Yamanova, M.; Ruksha, T.; Mikhutkina, S.; Trufanova, L. Disorders of folliculogenesis are associated with abnormal expression of peripheral benzodiazepine receptors in granulosa cells. Bull. Exp. Biol. Med. 2008, 145, 29–32. [Google Scholar] [CrossRef]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Pyne, S.G.; Bremner, J.B. An investigation into the cytotoxicity and mode of action of some novel N-alkyl-substituted isatins. J. Med. Chem. 2007, 50, 5109–5117. [Google Scholar] [CrossRef]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Wang, D.; Lin, C.-H.; Yang, H.-C.; Ma, Y.; Sargeant, A.; Chiu, C.-F.; Tsai, M.-H. A potent indole-3-carbinol–derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007, 67, 7815–7824. [Google Scholar] [CrossRef]
- Nicholson, D. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999, 6, 1028–1042. [Google Scholar]
- Cane, A.; Tournaire, M.-C.; Barritault, D.; Crumeyrolle-Arias, M. The endogenous oxindoles 5-hydroxyoxindole and isatin are antiproliferative and proapoptotic. Biochem. Biophys. Res. Commun. 2000, 276, 379–384. [Google Scholar] [CrossRef]
- Steinmetz, R.; Wagoner, H.A.; Zeng, P.; Hammond, J.R.; Hannon, T.S.; Meyers, J.L.; Pescovitz, O.H. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol. Endocrinol. 2004, 18, 2570–2582. [Google Scholar] [CrossRef]
- Georgakis, G.V.; Li, Y.; Rassidakis, G.Z.; Martinez-Valdez, H.; Medeiros, L.J.; Younes, A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin’s lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal–regulated kinase, and induces cell cycle arrest and cell death. Clin. Cancer Res. 2006, 12, 584–590. [Google Scholar] [CrossRef]
- Zhuang, S.; Schnellmann, R.G. A death-promoting role for extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 2006, 319, 991–997. [Google Scholar] [CrossRef]
- DiPaola, R.S. To arrest or not to G2-M cell-cycle arrest commentary re: AK Tyagi et al., silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest, and apoptosis. Clin. Cancer. Res. 2002, 8, 3311–3314. [Google Scholar]
- Singh, S.V.; Herman-Antosiewicz, A.; Singh, A.V.; Lew, K.L.; Srivastava, S.K.; Kamath, R.; Brown, K.D.; Zhang, L.; Baskaran, R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J. Biol. Chem. 2004, 279, 25813–25822. [Google Scholar] [CrossRef]
- Damiens, E.; Baratte, B.; Marie, D.; Eisenbrand, G.; Meijer, L. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: Induction of endoreplication following prophase arrest. Oncogene 2001, 20, 3786–3797. [Google Scholar] [CrossRef]
- Davis, S.T.; Benson, B.G.; Bramson, H.N.; Chapman, D.E.; Dickerson, S.H.; Dold, K.M.; Eberwein, D.J.; Edelstein, M.; Frye, S.V.; Gampe, R.T., Jr. Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors. Science 2001, 291, 134–137. [Google Scholar] [CrossRef]
- Lane, M.E.; Yu, B.; Rice, A.; Lipson, K.E.; Liang, C.; Sun, L.; Tang, C.; McMahon, G.; Pestell, R.G.; Wadler, S. A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001, 61, 6170–6177. [Google Scholar]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef]
- Marko, D.; Schätzle, S.; Friedel, A.; Genzlinger, A.; Zankl, H.; Meijer, L.; Eisenbrand, G. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 2001, 84, 283–289. [Google Scholar] [CrossRef]
- Jautelat, R.; Brumby, T.; Schäfer, M.; Briem, H.; Eisenbrand, G.; Schwahn, S.; Krüger, M.; Lücking, U.; Prien, O.; Siemeister, G. From the insoluble dye indirubin towards highly active, soluble CDK2-inhibitors. ChemBioChem 2005, 6, 531–540. [Google Scholar] [CrossRef]
- Eisenbrand, G.; Hippe, F.; Jakobs, S.; Muehlbeyer, S. Molecular mechanisms of indirubin and its derivatives: Novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004, 130, 627–635. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sandur, S.K.; Lin, X.; Chaturvedi, M.M.; Aggarwal, B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-κB signaling pathway. J. Biol. Chem. 2006, 281, 23425–23435. [Google Scholar]
- Adachi, J.; Mori, Y.; Matsui, S.; Takigami, H.; Fujino, J.; Kitagawa, H.; Miller Iii, C.A.; Kato, T.; Saeki, K.; Matsuda, T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 2001, 276, 31475–31478. [Google Scholar] [CrossRef]
- Spink, B.C.; Hussain, M.M.; Katz, B.H.; Eisele, L.; Spink, D.C. Transient induction of cytochromes P450 1A1 and 1B1 in MCF-7 human breast cancer cells by indirubin. Biochem. Pharmacol. 2003, 66, 2313–2321. [Google Scholar] [CrossRef]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Garaliene, V.; Welsh, W.; Arora, S.; Farruggia, G. Antitumor activity of new substituted 3-(5-Imidazo [2,1-b] thiazolylmethylene)-2-indolinones and study of their effect on the cell cycle 1. J. Med. Chem. 2005, 48, 5604–5607. [Google Scholar] [CrossRef]
- Chen, Z.; Merta, P.J.; Lin, N.-H.; Tahir, S.K.; Kovar, P.; Sham, H.L.; Zhang, H. A-432411, a novel indolinone compound that disrupts spindle pole formation and inhibits human cancer cell growth. Mol. Cancer Ther. 2005, 4, 562–568. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Young, F.M.; Phungtamdet, W.; Sanderson, B.J. Modification of MTT assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, WIL2NS. Toxicol. In Vitro 2005, 19, 1051–1059. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Esmaeelian, B.; Benkendorff, K.; Johnston, M.R.; Abbott, C.A. Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines. Mar. Drugs 2013, 11, 3802-3822. https://doi.org/10.3390/md11103802
Esmaeelian B, Benkendorff K, Johnston MR, Abbott CA. Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines. Marine Drugs. 2013; 11(10):3802-3822. https://doi.org/10.3390/md11103802
Chicago/Turabian StyleEsmaeelian, Babak, Kirsten Benkendorff, Martin R. Johnston, and Catherine A. Abbott. 2013. "Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines" Marine Drugs 11, no. 10: 3802-3822. https://doi.org/10.3390/md11103802
APA StyleEsmaeelian, B., Benkendorff, K., Johnston, M. R., & Abbott, C. A. (2013). Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines. Marine Drugs, 11(10), 3802-3822. https://doi.org/10.3390/md11103802