2. Results and Discussion
Compound
1 was isolated as a colorless amorphous solid with molecular formula of C
32H
54O
6 established by HR-ESI-MS, which showed a pseudo-molecular-ion peak at
m/z 557.3805 ((M + Na)
+ C
32H
54O
6Na
+; cacld. 557.3813), requiring six degrees of unsaturation. The IR absorption bands at 3427 and 1710 cm
−1 indicated the presence of OH and C=O groups, respectively, in agreement with the observations of the
13C NMR signals for four oxygenated C-atoms (δ
C 67.1, d; 71.0, d; 76.0, d and 76.6, s) and one C=O (δ
C 170.6, s). Its
13C NMR and DEPT spectra exhibited a total of 32 carbon signals classified into nine methyls (including one methoxyl), seven methylenes, ten methines (including four oxymethines), and six quaternary carbons (including one acetyl carbonyl and two olefinic carbons). These data together with the characteristic steroidal methyl signals at δ
H 0.85 (3H, d,
J = 6.6 Hz, Me-26), 0.89 (3H, d,
J = 6.6 Hz, Me-27), 0.78 (3H, d,
J = 6.6 Hz, Me-28), 0.67 (3H, d,
J = 6.6 Hz, Me-29), 0.92 (3H, s, Me-18), and 1.29 (3H, s, Me-19) (
Table 1), strongly suggested compound
1 to be a polyhydroxylated C29-steroid, similar to the nucleus of sarcsteroids A and B [
7] and to the side chain of 23,24-dimethylcholesta-17(20)-en-3β,5α,6β-triol [
6]. This speculation was supported by
1H,
1H-COSY and HMBC spectra as shown in
Figure 2 (
Supplementary Figures S9 and S11). The COSY correlations (H
2-1/H
2-2/H-3/H
2-4, H-6/H
2-7/H-8/H-9/H-11/H-12 and H-8/H-14/H
2-15/H-16), together with the key HMBC correlations of Me-19 with C-1 (δ
C 33.3), C-5 (δ
C 76.6), C-9 (δ
C 48.7) and C-10 (δ
C 39.9), Me-18 with C-12 (δ
C 43.7), C-13 (δ
C 43.9), C-14 (δ
C 51.0) and C-17 (δ
C 144.7), and H-16 (δ
H 4.08 (d,
J = 5.0 Hz)) with C-13 and C-17 could establish the 3,5,6-trihydroxy steroidal nucleus. HMBC correlations of H
3-OAc (δ
H 1.99 (s)) and H-11 (δ
H 5.16 (dt,
J = 12.1, 5.5 Hz)) with the carbonyl carbon signal (δ
C 170.6 s), and H
3-OMe with C-16 (δ
C 81.7) positioned the OAc and OMe groups at C-11 and C-16, respectively. The consecutive COSY corrections starting from H
2-22 (δ
H 2.07, 1.85) to H
3-29, together with HMBC correlations (
Figure 2) of H
3-21 with C-17, C-20 (δ
C 135.0) and C-22 (δ
C 41.9) further indicated the presence of a 23,24-dimethylcholesterol side chain connected to the mother nucleus through a double bond between C-17 and C-20. The planar structure of compound
1 was thus depicted as shown in
Figure 1.
Table 1.
1H and 13C NMR data (J and W1/2 values in Hz, δ in ppm) of compounds 1 and 3 in CDCl3 and 2 in C5D5N, J in Hz a.
Table 1.
1H and 13C NMR data (J and W1/2 values in Hz, δ in ppm) of compounds 1 and 3 in CDCl3 and 2 in C5D5N, J in Hz a.
| NO. | 1 | 2 | 3 |
|---|
| δH | δC | δH | δC | δH | δC |
|---|
| 1 | 1.18 m, 1.79 m | 33.3 | t | 2.97 m, 2.76 m | 35.4 | t | 3.63 br. s (W1/2 8.8) | 77.5 | d |
| 2 | 1.51 m, 1.81 m | 31.2 | t | 2.22 m, 2.37 m | 33.1 | t | 2.17 m, 1.82 m | 37.0 | t |
| 3 | 4.06 m (W1/2 24.0) | 67.1 | d | 4.92 m (W1/2 24.4) | 67.4 | d | 4.36 m (W1/2 22.0) | 63.8 | d |
| 4 | 1.62 m, 2.07 m | 41.1 | t | 2.41 m, 3.06 m | 44.0 | t | 1.78 m, 2.09 m | 41.3 | t |
| 5 | − | 76.6 | s | − | 76.5 | s | − | 77.2 | s |
| 6 | 3.56 br. s (W1/2 7.1) | 76.0 | d | 4.21 br. s | 76.8 | d | 3.51 br. s | 74.7 | d |
| 7 | 1.88 m, 1.68 m | 34.7 | t | 2.03 m, 2.44 m | 35.8 | t | 1.57 m, 1.91 m | 34.6 | t |
| 8 | 1.94 m | 28.1 | d | 2.33 m | 30.1 | d | 1.91 m | 29.5 | d |
| 9 | 1.81 m | 48.7 | d | 2.35 m | 53.1 | d | 2.21 m | 43.9 | d |
| 10 | − | 39.9 | s | − | 41.1 | s | − | 42.3 | s |
| 11 | 5.16 dt (12.1, 5.5) | 71.0 | d | 4.38 m | 68.5 | d | 5.18 dt (10.8, 5.3) | 72.4 | D |
| 12 | 2.65 dd (12.1, 5.5), 1.50 m | 43.7 | t | 1.70 m, 2.71 m | 53.0 | t | 2.43 dd (12.1, 5.3), 1.19 m | 46.0 | t |
| 13 | − | 43.9 | s | − | 43.5 | s | − | 42.9 | s |
| 14 | 1.79 m | 51.0 | d | 1.42 m | 55.9 | d | 1.26 m | 54.7 | d |
| 15 | 1.69 m, 1.21 m | 29.4 | t | 1.21 m, 1.73 m | 25.0 | t | 1.10 m, 1.64 m | 24.1 | t |
| 16 | 4.08 d (5.0) | 81.7 | d | 1.37 m, 2.05 m | 28.6 | t | 1.91 m, 1.31 m | 28.2 | t |
| 17 | − | 144.7 | s | 1.33 m | 58.4 | d | 1.17 m | 56.0 | d |
| 18 | 0.92 s | 16.7 | q | 0.81 s | 13.4 | q | 0.74 s | 12.7 | q |
| 19 | 1.29 s | 17.4 | q | 1.93 s | 17.7 | q | 1.20 s | 16.5 | q |
| 20 | − | 135.0 | s | 1.04 m | 35.6 | d | 1.38 m | 35.4 | d |
| 21 | 1.70 s | 18.1 | q | 1.09 d (6.6) | 21.3 | q | 0.91 d (6.4) | 18.6 | q |
| 22 | 2.07 m, 1.85 m | 41.9 | t | 0.28 m | 32.6 | d | 1.40 m, 1.04 m | 35.9 | t |
| 23 | 1.81 m | 32.4 | d | − | 25.0 | s | 1.86 m, 2.02 m | 24.7 | t |
| 24 | 1.05 m | 44.2 | d | 1.34 m | 49.7 | d | 5.07 t (7.4) | 125.0 | d |
| 25 | 1.57 m | 30.3 | d | − | 149.3 | s | | 131.1 | s |
| 26 | 0.85 d (6.6) | 22.0 | q | 4.87 s, 4.76 s | 110.3 | t | 1.59 s | 17.7 | q |
| 27 | 0.89 d (6.6) | 19.6 | q | 1.77 s | 23.6 | q | 1.68 s | 25.7 | q |
| 28 | 0.78 d (6.6) | 11.5 | q | 1.09 d (6.6) | 16.1 | q | − | − | |
| 29 | 0.67 d (6.6) | 13.5 | q | 0.85 s | 15.2 | q | − | − | |
| 30 | − | | | 0.55 dd (9.1, 4.1), −0.13 dd (5.7, 4.4) | 20.0 | t | − | − | |
| Ac | 1.99 s | 21.6 | q | − | − | | 2.04 s | 21.7 | q |
| | | 170.6 | s | − | − | | − | 169.3 | s |
| OMe | 3.27 s | 56.3 | q | − | − | | − | − | |
Figure 2.
1H, 1H-COSY and selected HMBC correlations of compound 1.
Figure 2.
1H, 1H-COSY and selected HMBC correlations of compound 1.
The relative configuration of the stereogenic centers for
1 was deduced from the proton-proton coupling constants (
Table 1) and NOESY spectrum (
Figure 3 and
Supplementary Figure S12). The half-peak-width of H-6 (δ
H 3.56 (br. s, W
1/2 = 7.1 Hz)) was indicative of its equatorial α-orientation, while the half-width of about 20.0 Hz for H-3 and the large coupling constants for H-11 (dd,
J = 12.1, 5.5 Hz) pointed to their axial α- and β-orientations, respectively [
8]. The NOESY cross peaks between Me-19 and Hβ-4 (δ
H 2.07), H-11 and H-8, between H-11 and Me-18, and between H-14 and H-9, H-16 and H-14 indicated their special relative orientations as shown in
Figure 3. The orientations of the OH groups, especially the tertiary OH group at C-5, were assigned by the pyridine-induced deshielding effect. The marked downfield shifts of H-3α and H
3-19β in pyridine-
d5 (δ
H-3 4.86, δ
Me-19 1.83) relative to CDCl
3 (δ
H-3 4.06, δ
Me-19 1.29) which was attributed to the 1,3-
syn-periplanar arrangement of hydroxyl groups (1,3 diaxial deshieding effect) were observed, indicating the orientations of OH-5α and OH-6β [
9]. The
Z geometry of the double bond Δ
17(20) was deduced from the NOESY cross peaks between Me-18 and Me-21, and between H-16 and H
2-22. In addition, the NOESY correlation of H-16 with H-14 but the lack of H-16 with H-18 indicated the α-orientation of H-16. Tentative assignments of 23
R and 24
R for the side chain of
1 were evident from the almost consistent
13C NMR data with the reported (23
R,24
R)-23,24-dimethylcholesta-17(20)-en-3β,5α,6β-triol [
6]. Accordingly, compound
1 was determined as (23
R,24
R,17
Z)-11α-acetoxy-16β-methoxy-23,24-dimethylcholest-17(20)-en-3β,5α,6β-triol, which was suspected to be an artifact from the isolation process.
Figure 3.
Key NOESY correlations for compound 1.
Figure 3.
Key NOESY correlations for compound 1.
The molecular formula of compound
2 was determined as C
30H
50O
4 from the HR-ESI-MS data ((M + Na)
+ 497.3614; calcd. 497.3601). In its 1D NMR spectra, six methyl proton signals (δ
H 0.81 (3H, s), 0.85 (3H, s), 1.93 (3H, s), 1.09 (6H, d,
J = 6.6 Hz), 1.77 (3H, s)) and four oxygenated carbon signals (δ
C 67.4 (d), 68.5 (d), 76.5 (s), 76.8 (d)), as well as a characteristic cyclopropane-bearing gorgosterol-type side chain (δ
H −0.13 (1H, dd,
J = 5.7, 4.4 Hz, H-30a), 0.28 (1H, m, H-22), 0.55 (1H, dd,
J = 9.1, 4.1 Hz, H-30b)) suggested
2 as a polyhydroxygorgosterol. Careful comparison the NMR data of
2 with those of the reported sarcoaldesterol A [
10] showed their closely structural similarity except that the isopropenyl group in side chain of
2 replaced the isopropyl group in sarcoaldesterol A. The HMBC correlations (
Supplementary Figure S20) of H-26 (δ
H 4.76, 4.87 (each 1H, s)) with C-24 (δ
C 49.7 (d)), C-25 (δ
C 149.3 (s)) and C-27 (δ
C 23.6 (q)) supported the above assumption. The relative configuration of
2 was assigned to be the same as sarcoaldesterol A, evidenced from NOESY spectrum (
Supplementary Figure S21) and similar NMR data together with biogenetic considerations. The structure of
2 was evidently established as (24
R)-gorgost-25-en-3β,5α,6β,11α-tetraol.
Molecular formula C
29H
48O
6 was assigned to compound
3 deduced from HR-ESI-MS at
m/
z 515.3357 ((M + Na)
+). It was similar to sarcsteroid B according to their
1H and
13C NMR spectra [
6,
7]. The marked difference was the presence of an additional OH group at C-1 in
3, which was confirmed by an oxygenated carbon signal (δ
C 77.5 (d, C-1)), a proton signal (δ
H 3.63 (br s, H-1)) and HMBC correlation (
Supplementary Figure S29) of H
3-19 (δ
H 1.20 (s)) with C-1. The half-peak-width of H-1 (br. s, W
1/2 = 8.8 Hz) and NOESY cross peak (
Supplementary Figure S30) between Me-19 and H-1 were indicative of its equatorial β-orientation. Thus, compound
3 was identified as 11α-acetoxycholest-24-en-1α,3β,5α,6β-tetraol.
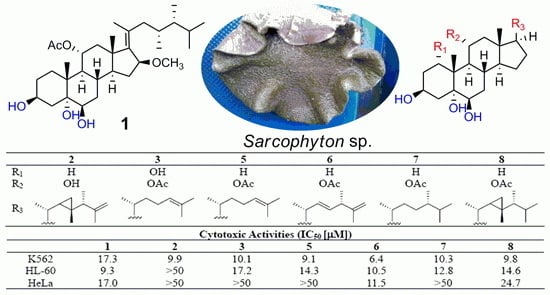
Seven known analogs (
Figure 1), (24
R)-methylcholest-7-en-3β,5α,6β-triol (
4) [
11], 11α-acetoxy-cholest-24-en-3β,5α,6β-triol (
5) [
6,
7], (22
E,24
S)-11α-acetoxy-ergost-22,25-dien-3β,5α,6β-triol (
6) [
6,
7], (24
S)-11α-acetoxy-ergost-3β,5α,6β-triol (
7) [
12], (24
R)-11α-acetoxy-gorgost-3β,5α,6β-triol (
8) [
6,
7], (24
S)-ergost-3β,5α,6β, 11α-tetraol (
9) [
10] and (24
S)-23,24-dimethylcholest-22-en-3β,5α,6β,11α-tetraol (
10) [
6,
7] were also isolated and identified by comparison of their spectroscopic data with those reported in the literature.
Because of the cytotoxicities [
13,
14] and antiviral activities [
15] for some reported polyhydroxylated steroids, the cytotoxicities for all the compounds (
1–
10) against three tumor cell lines (human leukemia, K562; human myeloid leukemia, HL-60; human cervical carcinoma, HeLa) were evaluated
in vitro by MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-2H-tetrazolium bromide) and SRB (Sulforhodamine B) methods (
Table 2). Compounds
1,
3, and
5–
8, which shared the same nucleus substituted by an OAc group at C-11, showed potent cytotoxicities against certain cell lines with IC
50 values less than 10.3 μM, while compounds
4 without substitution,
9 and
10 with OH substitution at C-11 were inactive against tested cell lines, indicating the 11-OAc group to be a very important pharmacophore. Compound
2 with an OH group at C-11 showed selective inhibitory activity only against K562 cell line with IC
50 value of 9.9 μM, compounds
9 and
10 with the same substitution also showed the similar selectivity, implying that the OH substitution at C-11 could improve the selectivity but decrease activity. Meanwhile, compounds
1,
6, and
8 showed broad-spectrum activities against three cell lines. The comparative analysis of the cytotoxic data for compounds
3 and
5 indicated that the 1-OH substitution did not contribute to the activity.
Table 2.
Cytotoxicity data (IC50 in μM) for compounds 1–10.
Table 2.
Cytotoxicity data (IC50 in μM) for compounds 1–10.
| Compounds | K562 a | HL-60 a | HeLa b |
|---|
| 1 | 17.3 | 9.3 | 17.0 |
| 2 | 9.9 | >50 | >50 |
| 3 | 10.1 | 17.2 | >50 |
| 4 | 21.7 | 33.7 | >50 |
| 5 | 9.1 | 14.3 | >50 |
| 6 | 6.4 | 10.5 | 11.5 |
| 7 | 10.3 | 12.8 | >50 |
| 8 | 9.8 | 14.6 | 24.7 |
| 9 | 24.5 | 32.5 | >50 |
| 10 | 26.6 | >50 | >50 |
| ADM(Adriamycin) c | 0.2 | 0.02 | 0.6 |
Antiviral activities of all the isolates against H1N1 IAV were also evaluated by the cytopathic effects assays (CPE). Only compounds
4 and
9, despite of their lack of cytotoxicity in the above experiments, exhibited significant anti-H1N1 virus activity with IC
50 values of 19.6 and 36.7 μg/mL (Ribavirin was used as a positive control with IC
50 value of 24.6 μg/mL), respectively. This is the first report of a specific type of steroid having activity against a flu virus, except for the one report that a similar type of steroid had activity against human cytomegalovirus [
15].
3. Experimental Section
3.1. General Methods
Optical rotations were measured with a JASCO P-1020 polarimeter (JASCO Corporation, Tokoyo, Japan). UV spectra were measured with a Beckman DU640 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA). IR spectra were recorded on a Nicolet NEXUS 470 spectrophotometer (International Equipment Trading Ltd., Vernon Hills, IL, USA). NMR spectra were measured with a JEOL JNMECP 600 (JEOL Ltd., Tokoyo, Japan) and Bruker 600 spectrometers (Bruker Daltonics Inc., Billerica, MA, USA). The melting points uncorrected were measured on an X-4 micro-scopic melting point apparatus (Shanghai Instrument Physical Optics Instrument Co. Ltd., Shanghai, China). Chemical shifts were referenced to residual non deuterated solvent signals (CDCl3: δH 7.26 ppm, δC 77.0 ppm; C5D5N: δH 7.21, 7.58, 8.73 ppm, δC 123.5, 135.5, 149.9 ppm). HR-ESI-MS data were obtained on a Micromass Q-Tof Ultima GLOBAL GAA076 LC mass spectrometer on a Thermo Scientific LTQ orbitrap XL mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). HPLC isolation was achieved on a Waters 2695 instrument using a semi-preparative HPLC column (YMC-packed C18, 5 μm, 250 × 10 mm) (YMC Co. Ltd., Kyoto, Japan). Vacuum liquid chromatography (VLC) was performed on special chromatographic column utilizing reduced pressure to increase the flow rate of the mobile phase. Silica-gel (200–300 mesh, 300–400 mesh, Qingdao Marine Chemical Factory, Qingdao, Shandong, China) and ODS silica-gel (50 μm, Merck, Darmstadt, Germany) were used for column chromatography (CC). TLC was carried out with glass precoated silica gel GF254 plates (Qingdao Marine Chemical Factory, Qingdao, Shandong, China). Spots were visualized under UV light or by spraying with 10% H2SO4 in EtOH-H2O (95:5, v/v) followed by heating.
3.2. Animal Material
The Sarcophyton sp. was collected from the South Sea (Weizhou Islands sea area) at a depth of 12 m. The specimen was identified by Professor Zou, R.L. (South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China). The voucher specimen (NO. WZD-2010-03) was deposited at State Key Laboratory of Marine Drugs, Ocean University of China, Qingdao, Shandong, China.
3.3. Extraction and Isolation
The frozen sample of Sarcophyton sp. (5.0 kg, wet weight) was homogenized and then extracted with MeOH for three times (5 L × 3, each, 3 days) at room temperature, and the solution was evaporated in vacuum to yield a crude extract (70.0 g) which was subjected to column chromatography (CC) on silica gel using petroleum ether/acetone (from 100:1 to 1:2, v/v) as eluent to obtain seven fractions (Fr. 1–Fr. 7). Each fraction was detected by TLC and was preliminarily bio-assayed for cytotoxicities, and Fr. 6 was found to be the most active fraction containing the main polyhydroxylated steroids of this species. Thus, Fr. 6 (4.58 g) was subjected to Sephadex LH-20 (MeOH) and was further separated by repeat chromatography on a silica gel column eluting with petroleum ether/acetone (from 3:1 to 1:1) to afford four sub-fractions (Fr. 6.1–Fr. 6.4). Fr. 6.2 (68.1 mg) was purified by ODS column chromatograph with a gradient increasing MeOH in H2O (from 30% to 85%) to yield Fr. 6.2.1–Fr. 6.2.3. Fr. 6.2.3 (30 mg) was further separated by HPLC (ODS. C18; 90% MeOH in H2O) to yield compound 4 (2.8 mg). Fr. 6.3 (558.5 mg) was applied to an ODS column and eluted with MeOH in H2O (from 30% to 85%) to obtain Fr. 6.3.1–Fr. 6.3.4. Fr. 6.3.2 (61.4 mg) was further purified by HPLC (ODS. C8; 80% MeOH in H2O) to yield compound 5 (4.1 mg) and compound 6 (2.2 mg). Fr. 6.3.3 (255.1 mg) was further separated to three fractions (Fr. 6.3.3.1–Fr. 6.3.3.3) by Sephadex LH-20 (petroleum ether/CH2Cl2/MeOH 2:2:1). Fr. 6.3.3.1 (29.1 mg) was repurified with HPLC (ODS. C18; 83% MeOH in H2O with 1‰ HCOOH) to afford compound 7 (4.9 mg). Fr. 6.3.4 (105.6 mg) was also purified by HPLC (ODS. C8; 85% MeOH in H2O with 1‰ HCOOH) to yield compound 8 (37.3 mg). Fr. 6.4 (1.0 g) was submitted to a silica gel column eluting with CH2Cl2/MeOH/H2O (300:10:3.3) to afford two fractions (Fr. 6.4.1 and Fr. 6.4.2). Fr. 6.4.1 (109.1 mg) was further separated by HPLC (ODS. C18; 77% MeOH in H2O) to yield compound 3 (4.2 mg) and Fr. 6.4.2 (33.2 mg) was purified by HPLC (ODS. C8; 80% MeOH in H2O with 1‰ HCOOH) to yield compounds 1 (3 mg), 2 (5.1 mg), 9 (1.9 mg) and 10 (8.7 mg).
Compound 1: (23
R,24
R,17
Z)-11α-acetoxy-16β
-methoxy-23,24-dimethylcholest-17(20)-en-3β,5α,6β-triol. Colorless amorphous solid; melting point (m.p.) 234–236 °C;
![Marinedrugs 11 04788 i001]()
= −67.1 (
c = 2.7, CHCl
3); IR (KBr): 3427, 2960, 2873, 1731, 1710, 1377, 1250, 1068, 1025 cm
−1;
1H and
13C NMR: see
Table 1; HR-ESI-MS:
m/z 557.3805 [M + Na]
+ (calcd. for C
32H
54NaO
6+, 557.3813).
Compound 2: (24
R)-gorgost-25-en-3β,5α,6β,11α-tetraol. Colorless amorphous solid; m.p. 261–262 °C;
![Marinedrugs 11 04788 i001]()
= −2.69 (
c = 3.2, MeOH); IR (KBr): 3422, 2925, 1702, 1459, 1378, 1261, 1097, 1032 cm
−1;
1H and
13C NMR: see
Table 1; HR-ESI-MS: 497.3614 [M + Na]
+ (calcd. for C
30H
50NaO
4+, 497.3601).
Compound 3: 11α-acetoxycholest-24-en-1α,3β,5α,6β-tetraol. Colorless amorphous solid; m.p. 266–268 °C;
![Marinedrugs 11 04788 i001]()
= −17.82 (
c = 3.9, CHCl
3); IR (KBr): 3394, 2926, 2889, 1727, 1378, 1257, 1068, 1040 cm
−1;
1H and
13C NMR: see
Table 1; HR-ESI-MS: 515.3357 [M + Na]
+ (calcd. for C
29H
48NaO
6+, 515.3343).
3.4. Cytotoxic Assay
In vitro cytotoxicities were determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay [
16] against K562 (human leukemia cells) and HL-60 (human myeloid leukemia cells), SRB (Sulforhodamine B) assay [
17] against HeLa (human cervical carcinoma cells). All the cell lines were purchased from Shanghai Institute of Cell Biology (Shanghai, China). Cytotoxic data (
Table 2) for compounds
1–
10 were obtained on the basis of five concentrations with three replications. Adriamycin (doxorubicin, ADM) was used as a positive control, and IC
50 values >50 μM were considered to be inactive in cytotoxic assays.
In the MTT assay, the cells were cultured in RPMI-1640 supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Those cell suspensions (200 μL) at a density of 5 × 104 cells/mL were plated in 96-well microtiter plates and incubated for 24 h at the above conditions. The test compound solution (2 μL in DMSO) at different concentrations in triplicate was added to each well and further incubated for 72 h under the same conditions. 20 μL of the MTT solution (5 mg/mL in IPMI-1640 medium) was then added to each well and incubated for 4 h. The old medium containing MTT (150 μL) was then gently replaced by DMSO and vibrated to dissolve any formazan [1-(4-Iodophenyl)-5-(4-nitrophenyl)-3-phenylformazan] crystals formed. The optical density of the solution was measured on a Spectra Max Plus plate reader at 570 nm. The IC50 value of each compound was calculated by Reed and Muench’s method.
In the SRB assay, 200 μL of the cell suspensions were plated in 96-well plates at a density of 2 × 105 cell mL−1. Then 2 μL of the test solutions (in MeOH) was added to each well, and the culture was further incubated for 24 h. The cells were fixed with 12% trichloroacetic acid, and the cell layer was stained with 0.4% SRB. The absorbance of the SRB solution was measured at 515 nm. Dose-response curves were generated, and the IC50 values (the concentration of compound required to inhibit cell proliferation by 50%) were calculated from the linear portion of log dose-response curves.
3.5. Anti-H1N1 Virus Assay
The antiviral activity against H1N1 was evaluated by the CPE inhibition assay [
18]. Confluent MDCK cell monolayers were firstly incubated with influenza virus (A/Puerto Rico/8/34 (H1N1), PR/8) at 37 °C for 1 h. After removing the virus dilution, cells were maintained in infecting media (RPMI 1640, 4 μg/mL of trypsin) containing different concentrations of test compounds at 37 °C. After 48 h incubation at 37 °C, cells were fixed with 100 μL of 4% formaldehyde for 20 min at room temperature. After removal of the formaldehyde, the cells were stained with 0.1% crystal violet for 30 min. The plates were washed and dried, and the intensity of crystal violet staining for each well was measured in a microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. The IC
50 was calculated as the compound concentration required inhibiting CPE production at 48 h post-infection by 50%. Ribavirin (LuKang Cisen (Jining, China)) was used as positive control, and compounds with an inhibition rate of >70%, >50%, and <30% at 50 μg/mL were respectively regarded having strong, moderate, and weak activities.



 = −67.1 (c = 2.7, CHCl3); IR (KBr): 3427, 2960, 2873, 1731, 1710, 1377, 1250, 1068, 1025 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: m/z 557.3805 [M + Na]+ (calcd. for C32H54NaO6+, 557.3813).
= −67.1 (c = 2.7, CHCl3); IR (KBr): 3427, 2960, 2873, 1731, 1710, 1377, 1250, 1068, 1025 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: m/z 557.3805 [M + Na]+ (calcd. for C32H54NaO6+, 557.3813). = −2.69 (c = 3.2, MeOH); IR (KBr): 3422, 2925, 1702, 1459, 1378, 1261, 1097, 1032 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: 497.3614 [M + Na]+ (calcd. for C30H50NaO4+, 497.3601).
= −2.69 (c = 3.2, MeOH); IR (KBr): 3422, 2925, 1702, 1459, 1378, 1261, 1097, 1032 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: 497.3614 [M + Na]+ (calcd. for C30H50NaO4+, 497.3601). = −17.82 (c = 3.9, CHCl3); IR (KBr): 3394, 2926, 2889, 1727, 1378, 1257, 1068, 1040 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: 515.3357 [M + Na]+ (calcd. for C29H48NaO6+, 515.3343).
= −17.82 (c = 3.9, CHCl3); IR (KBr): 3394, 2926, 2889, 1727, 1378, 1257, 1068, 1040 cm−1; 1H and 13C NMR: see Table 1; HR-ESI-MS: 515.3357 [M + Na]+ (calcd. for C29H48NaO6+, 515.3343).