Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation of Fumigaclavine C

2.2. Anti-Proliferative Effect of Fumigaclavine C on MCF-7 Cells
2.3. Effects of Fumigaclavine C on Migration and Invasion of MCF-7 Cells
2.4. Effect of Fumigaclavine C on MMP-2 and -9 Expressions in MCF-7 Breast Cancer Cells

2.5. Effect of Fumigaclavine C on ERK, JNK, and p38 MAPK Signaling Pathway

2.6. The Effects of Fumigaclavine C on the Cell Cycle of MCF-7 Cells

2.7. Effect of Fumigaclavine C on p53 Family Gene Expression in MCF-7 Breast Cancer Cells
2.8. Effect of Fumigaclavine C on Cyclin B1, Cyclin E, CDK2, and CDK4 Expression in MCF-7 Breast Cancer Cells
2.9. The Morphological Changes and DNA Damages of MCF-7 Cells Observed with Hoechst 33258 Staining
2.10. Fumigaclavine C Induced DNA Fragmentation in MCF-7 Cells

2.11. Effect of Fumigaclavine C on PI3K/Akt Pathway in MCF-7 Breast Cancer Cells
2.12. Effect of Fumigaclavine C on Bcl-2 Family Protein Expression in MCF-7 Breast Cancer Cells
2.13. Effect of Fumigaclavine C on Caspase-3, -8, and -9 Expression in MCF-7 Breast Cancer Cells

2.14. Effect of Fumigaclavine C on Cytochrome C and Apaf-1 Protein Expression in MCF-7 Breast Cancer Cells

2.15. Effect of Fumigaclavine C on p50, p65 and IKK Expression in MCF-7 Breast Cancer Cells
2.16. Computational Modeling of the Fumigaclavine C Apoptosis Activity

| Est. Free Energy of Binding | Est. Ingibition Constant, Ki | vdW + Hbond + desolv Energy | Electrostatic Energy | Total Internoiec. Energy | Frequency |
|---|---|---|---|---|---|
| −6.97 kcal/mol | 7.75 μM | −6.74 kcal/mol | −0.92 kcal/mol | −7.66 kcal/mol | 40% |
| −6.60 kcal/mol | 14.57 μM | −7.24 kcal/mol | −0.11 kcal/mol | −7.35 kcal/mol | 10% |
| −6.56 kcal/mol | 15.59 μM | −7.06 kcal/mol | −0.23 kcal/mol | −7.29 kcal/mol | 10% |
| −6.34 kcal/mol | 22.62 μM | −6.78 kcal/mol | −0.29 kcal/mol | −7.07 kcal/mol | 30% |
| −5.85 kcal/mol | 51.78 μM | −6.26 kcal/mol | −0.30 kcal/mol | −6.55 kcal/mol | 10% |
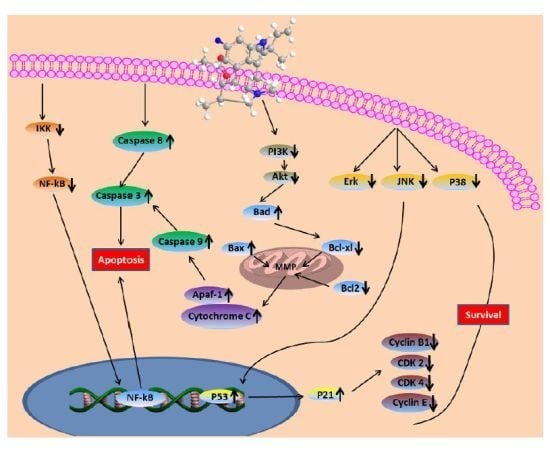
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. Cell Viability (MTT) Assay
4.6. Cell Migration and Invasion Assay
4.7. Hoechst 33258 Staining Assay
4.8. Cell Cycle Assay
4.9. DNA Laddering Assay
4.10. Reverse Transcription-PCR (Polymerase Chain Reaction)
4.11. Western Blot Analysis
4.12. Docking Calculations
5. Conclusions

Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Thomas, A.; Murry, T.; Thun, M. Cancer statistics. CA Cancer J. Clin. 2002, 52, 23–37. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics. CA. Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From chemoprevention to chemotherapy: Common targets and common goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Xin, Z.H.; Fang, Y.C.; Du, L.; Zhu, T.J.; Duan, L.; Chen, J.; Gu, Q.Q.; Zhu, W.M. Aurantiomides A–C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J. Nat. Prod. 2007, 70, 853–855. [Google Scholar] [CrossRef]
- Osterhage, C.; Kaminsky, R.; Konig, G.M.; Wright, A.D. Ascosali-pyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J. Org. Chem. 2000, 65, 6412–6417. [Google Scholar] [CrossRef]
- Lu, X.H.; Shi, Q.W.; Zheng, Z.H.; Ke, A.B.; Zhang, H.; Huo, C.H.; Ma, Y.; Ren, X.; Li, Y.Y.; Lin, J.; et al. Oxepinamides: Novel liver X receptor agonists from Aspergillus puniceus. Eur. J. Org. Chem. 2011, 2011, 802–807. [Google Scholar]
- Du, L.; Li, D.H.; Zhu, T.J.; Cai, S.X.; Wang, F.P.; Xiao, X.; Gu, Q.Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Fallon, J.P.; Reeves, E.P.; Kavanagh, K. Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J. Med. Microbiol. 2010, 59, 625–633. [Google Scholar] [CrossRef]
- Fujiki, H.; Mori, M.; Nakayasu, M.; Terada, M.; Sugimura, T.; Mooret, R.E. Indole alkaloids: Dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc. Natl. Acad. Sci. USA 1981, 78, 3872–3876. [Google Scholar] [CrossRef]
- Ge, H.M.; Yu, Z.G.; Zhang, J.; Wu, J.H.; Tan, R.X. Bioactive alkaloids from endophytic Aspergillus fumigatus. J. Nat. Prod. 2009, 72, 753–755. [Google Scholar] [CrossRef]
- Shu, R.G.; Wang, F.W.; Yang, Y.M.; Liu, Y.X.; Tan, R.X. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, an endophytic fungus in Quercus variabilis. Lipids 2004, 39, 667–673. [Google Scholar] [CrossRef]
- Lu, H.; Zou, W.X.; Meng, J.C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000, 151, 67–73. [Google Scholar] [CrossRef]
- Cole, R.J.; Kirksey, J.W.; Dorner, J.W.; Wilson, D.V.; Johnson, J.C.; Bedell, D. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. J. Agric. Food Chem. 1977, 25, 826–830. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Wang, J.; Wang, L.; Yin, H.; Tan, R. Fumigaclavine C improves concanavalin A-induced liver injury inmicemainly via inhibiting TNF-α production and lymphocyte adhesion to extracellular matrices. J. Pharm. Pharmacol. 2004, 56, 775–784. [Google Scholar] [CrossRef]
- Kuramoto, M.; Arimoto, H.; Uemura, D. Bioactive alkaloids from the sea: a review. Mar. Drugs 2004, 2, 39–54. [Google Scholar]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar]
- Hetz, C.A.; Torres, V.; Quest, A.F. Beyond apoptosis: nonapoptotic cell death inphysiology and disease. Biochem. Cell Biol. 2005, 83, 579–588. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Williams, G.T.; Smith, C.A. Molecular regulation of apoptosis: genetic controls on cell death. Cell 1993, 74, 777–779. [Google Scholar] [CrossRef]
- Pereira, D.M.; Cheel, J.; Areche, C.; San-Martin, A.; Rovirosa, J.; Silva, L.R.; Valentao, P.; Andrade, P.B. Anti-proliferative activity of meroditerpenoids isolated from the brown alga Stypopodium flabelliforme against several cancer cell lines. Mar. Drugs 2011, 9, 852–862. [Google Scholar] [CrossRef]
- Du, R.H.; Li, E.G.; Cao, Y.; Song, Y.C.; Tan, R.X. Fumigaclavine C inhibits tumor necrosis factor α production via suppression of toll-like receptor 4 and nuclear factor κB activation in macrophages. Life Sci. 2011, 89, 235–240. [Google Scholar] [CrossRef]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008, 78, 356–365. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Yang, G.H.; Jarvis, B.B.; Chung, Y.J.; Pestka, J.J. Apoptosis Induction by the satratoxins and other trichothecene mycotoxins: Relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000, 164, 149–160. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar]
- Tibbles, L.A.; Woodgett, J.R. The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 1999, 55, 1230–1254. [Google Scholar] [CrossRef]
- Tournier, C.; Hess, P.; Yang, D.D.; Xu, J.; Turner, T.K.; Nimnual, A.; Bar-Sagi, D.; Jones, S.N.; Flavell, R.A.; Davis, R.J. Requirement of JNK for stress-induced activation of the cytochrome C-mediated death pathway. Science 2000, 288, 870–874. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Wu, G.S. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004, 2, 156–161. [Google Scholar]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Wang, J.H.; Yu, B.; He, P.; Bai, X. Roles of Bcl-2 family members, PI3K and NF-κB pathways in Escherichia coli-induced apoptosis in human monocytic U937 cells. World J. Microb. Biot. 2011, 27, 1827–1838. [Google Scholar] [CrossRef]
- Lin, M.L.; Lu, Y.C.; Chung, J.G.; Wang, S.G.; Lin, H.T.; Kang, S.E.; Tang, C.H.; Ko, J.L.; Chen, S.S. Down-regulation of MMP-2 through the p38 MAPK-NF-κB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol. Carcinog. 2010, 49, 783–797. [Google Scholar]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Rahman, K.W.; Ali, S.; Aboukameel, A.; Sarkar, S.H.; Wang, Z.; Philip, P.A.; Sakr, W.A.; Raz, A. Inactivation of NF-κB by 3, 3′-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol. Cancer Ther. 2007, 6, 2757–2765. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Cahir-McFarland, E.D.; Davidson, D.M.; Schauer, S.L.; Duong, J.; Kieff, E. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-trans formed lympho blastoid cells. Proc. Natl. Acad. Sci. USA 2000, 97, 6055–6060. [Google Scholar] [CrossRef]
- Baldwin, A.S.J. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef]
- Beg, A.A.; Baldwin, A.S.J. The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993, 7, 2064–2070. [Google Scholar] [CrossRef]
- Liao, Y.F.; Rao, Y.K.; Tzeng, Y.M. Aqueous extract of Anisomeles indica and its purified compound exerts anti-metastatic activity through inhibition of NF-κB/AP-1-dependent MMP-9 activation in human breast cancer MCF-7 cells. Food Chem. Toxicol. 2012, 50, 2930–2936. [Google Scholar] [CrossRef]
- Roy, N.; Deveraux, O.L.; Takahashi, R.; Salvesen, G.S.; Reed, J.C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997, 16, 6914–6925. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Reed, J.C. IAP family proteins suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Huang, Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene 2000, 19, 6627–6631. [Google Scholar] [CrossRef]
- Antonsson, B. Bax and other pro-apoptotic Bcl-2 family ‘‘killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 2001, 306, 347–361. [Google Scholar] [CrossRef]
- Zornig, M.; Hueber, A.; Baum, W.; Evan, G. Apoptosis regulators and their role in tumorigenesis. Biochem. Biophys. Acta 2001, 1551, 1–37. [Google Scholar]
- Leist, M.; Jaattela, M. Four deaths and a funeral: From caspases to alternative mechanism. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef]
- Nagy, G.; Szarka, A.; Lotz, G.; Dóczi, J.; Wunderlich, L.; Kiss, A.; Jemnitz, K.; Veres, Z.; Bánhegyi, G.; Schaff, Z.; et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 2010, 243, 96–103. [Google Scholar] [CrossRef]
- Zou, H.; Yang, R.M.; Hao, J.S.; Wang, J.; Sun, C.H.; Fesik, S.W.; Wu, J.C.; Tomaselli, K.J.; Armstrong, R.C. Regulation of the apaf-1/caspase-9 apoptosome bycaspase-3 and XIAP. J. Biol. Chem. 2003, 278, 8091–8098. [Google Scholar] [CrossRef]
- Kuida, K.; Haydar, T.F.; Kuan, C.Y.; Gu, Y.; Taya, C.J.; Karasuyama, H.; Su, M.S.; Rakic, P.; Flavell, R.A. Reduced apoptosis and cytochrome C-mediated caspase activation in mice lacking caspase 9. Cell 1998, 94, 325–337. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Jin, U.H.; Suh, S.J.; Chang, H.W.; Son, J.K.; Lee, S.H.; Son, K.H.; Chang, Y.C.; Kim, C.H. Tanshinone IIA from salvia miltiorrhiza bungeinhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J. Cell. Biochem. 2008, 104, 15–26. [Google Scholar] [CrossRef]
- Frisk, T.; Rydholm, S.; Liebmann, T.; Svahn, H.A.; Stemme, G.; Brismar, H. A microfluidic device for parallel 3-D cell cultures in asymmetric environments. Electrophoresis 2007, 28, 4705–4712. [Google Scholar] [CrossRef]
- Yin, X.Y.; Grove, L.; Datta, N.S.; Long, M.W.; Prochownik, E.V. C-myc overexpression and p53 loss cooperate to promote genomic instability. Oncogene 1999, 18, 1177–1184. [Google Scholar] [CrossRef]
- Shinzawa, K.; Watanabe, Y.; Akaike, T. Primary cultured murine hepatocytes but not hepatoma cells regulate the cell number through density-dependent cell death. Cell Death Differ. 1995, 2, 133–140. [Google Scholar]
- Roth, M.J.; Tanese, N.; Goff, S.P. Purification and characterization of murine retroviral reverse transcriptase expressed in Escherichia coli. J. Biol. Chem. 1985, 260, 9326–9335. [Google Scholar]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform. 2009, 1, 15. [Google Scholar] [CrossRef]
- Halgren, E.; Marinkovic, K.; Chauvel, P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 156–164. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a lamarckian genetic algorithm and and empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Solis, F.J.; Wets, R.J.B. Minimization by Random Search Techniques. Math. Oper. Res. 1981, 6, 19–30. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.-X.; Himaya, S.W.A.; Dewapriya, P.; Zhang, C.; Kim, S.-K. Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells. Mar. Drugs 2013, 11, 5063-5086. https://doi.org/10.3390/md11125063
Li Y-X, Himaya SWA, Dewapriya P, Zhang C, Kim S-K. Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells. Marine Drugs. 2013; 11(12):5063-5086. https://doi.org/10.3390/md11125063
Chicago/Turabian StyleLi, Yong-Xin, S.W.A. Himaya, Pradeep Dewapriya, Chen Zhang, and Se-Kwon Kim. 2013. "Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells" Marine Drugs 11, no. 12: 5063-5086. https://doi.org/10.3390/md11125063
APA StyleLi, Y. -X., Himaya, S. W. A., Dewapriya, P., Zhang, C., & Kim, S. -K. (2013). Fumigaclavine C from a Marine-Derived Fungus Aspergillus Fumigatus Induces Apoptosis in MCF-7 Breast Cancer Cells. Marine Drugs, 11(12), 5063-5086. https://doi.org/10.3390/md11125063