Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp.
Abstract
:1. Introduction

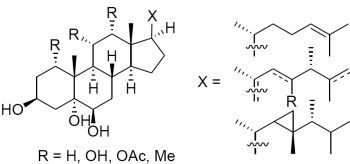
2. Results and Discussion
| Carbon | 1 a | 2 a | 3 b | 4 a | 5 a | 6 a | 7 a |
|---|---|---|---|---|---|---|---|
| 1 | 33.5 t | 33.5 t | 76.8 d | 34.4 t | 32.3 t | 33.7 t | 33.9 t |
| 2 | 31.3 t | 31.3 t | 37.2 t | 31.2 t | 30.9 t | 31.3 t | 31.2 t |
| 3 | 67.2 d | 67.2 d | 64.2 d | 67.4 d | 67.6 d | 67.1 d | 67.5 d |
| 4 | 41.3 t | 41.3 t | 41.3 t | 41.0 t | 40.8 t | 41.3 t | 41.3 t |
| 5 | 76.6 s | 76.6 s | 76.8 s | 76.7 s | 76.7 s | 76.6 s | 76.7 s |
| 6 | 76.1 d | 76.1 d | 75.2 d | 76.1 d | 76.1 d | 76.0 d | 76.0 d |
| 7 | 34.8 t | 34.9 t | 35.2 t | 34.5 t | 34.8 t | 34.4 t | 34.0 t |
| 8 | 29.4 d | 29.4 d | 29.4 d | 29.1 d | 28.9 d | 29.4 d | 28.9 d |
| 9 | 48.6 d | 48.7 d | 46.6 d | 53.0 d | 45.9 d | 42.7 d | 46.3 d |
| 10 | 39.9 s | 39.9 s | 42.1 s | 40.0 s | 38.4 s | 39.6 s | 39.6 s |
| 11 | 71.1 d | 71.1 d | 68.0 d | 68.7 d | 21.5 t | 73.4 d | 70.7 d |
| 12 | 46.4 t | 46.3 t | 50.9 t | 51.8 t | 37.9 t | 75.0 d | 80.5 d |
| 13 | 42.9 s | 42.7 s | 43.2 s | 43.1 s | 44.7 s | 46.7 s | 46.0 s |
| 14 | 54.6 d | 54.7 d | 54.7 d | 55.1 d | 52.2 d | 45.3 d | 47.4 d |
| 15 | 24.2 t | 24.2 t | 24.2 t | 24.2 t | 72.2 d | 23.9 t | 23.8 t |
| 16 | 28.2 t | 28.5 t | 28.2 t | 28.0 t | 34.4 t | 27.5 t | 27.6 t |
| 17 | 56.0 d | 55.9 d | 55.8 d | 56.7 d | 149.0 s | 49.2 d | 50.1 d |
| 18 | 12.7 q | 12.9 q | 12.8 q | 13.4 q | 17.3 q | 11.8 q | 12.7 q |
| 19 | 16.8 q | 16.8 q | 16.3 q | 17.0 q | 16.8 q | 16.7 q | 16.7 q |
| 20 | 35.4 d | 39.8 d | 36.1 d | 34.5 d | 133.0 s | 35.1 d | 34.6 d |
| 21 | 18.6 q | 20.7 q | 18.9 q | 20.6 q | 17.1 q | 20.1 q | 20.6 q |
| 22 | 35.9 t | 135.5 d | 33.6 t | 131.3 d | 42.0 t | 31.9 d | 31.9 d |
| 23 | 24.7 t | 131.8 d | 30.6 t | 135.7 s | 32.7 d | 25.9 s | 25.9 s |
| 24 | 125.1 d | 43.6 d | 39.1 d | 50.2 d | 44.2 d | 50.8 d | 50.8 d |
| 25 | 131.1 s | 149.8 s | 31.5 d | 30.8 d | 30.1 d | 32.0 d | 32.0 d |
| 26 | 17.6 q | 108.8 t | 20.5 q | 21.7 q | 21.6 q | 21.5 q | 21.5 q |
| 27 | 25.7 q | 20.6 q | 17.6 q | 20.1 q | 19.1 q | 22.2 q | 21.3 q |
| 28 | 18.9 q | 15.5 q | 17.0 q | 11.5 q | 15.4 q | 15.5 q | |
| 29 | 13.3 q | 13.8 q | 14.3 q | 14.3 q | |||
| 30 | 21.3 t | 21.3 t | |||||
| COOCH3 | 22.1 q | 22.1 q | 22.1 q | 22.2 q | |||
| COOCH3 | 170.4 s | 170.4 s | 170.0 s | 170.4 s |
| Position | 1 a | 2 a | 3 b | 4 a |
|---|---|---|---|---|
| 1 | 1.26 (m), 1.81( m) | 1.22 (m), 1.76 (m) | 4.11 (br s) | 1.50 (m), 1.71 (m) |
| 2 | 1.47 (m), 1.82 (m) | 1.53 (m), 1.77 (m) | 1.86 (m), 2.18 (m) | 1.56 (m), 1.86 (m) |
| 3 | 4.05 (m) | 4.05 (m) | 4.40 (m) | 4.07 (m) |
| 4 | 1.62 (ov), 2.07 (t, 12.2) | 1.58 (ov), 2.08 (d, 11.8) | 1.77 (ov), 2.08 (ov) | 1.67 (ov), 2.11 (ov) |
| 6 | 3.52 (br s) | 3.52 (br s) | 3.51 (br s) | 3.53 (br s) |
| 7 | 1.61 (m), 1.76 (m) | 1.58 (m), 1.74 (m) | 1.52 (m), 1.88 (m) | 1.73 (m), 2.08 (m) |
| 8 | 1.87 (m) | 1.76 (m) | 1.88 (m) | 1.76 (m) |
| 9 | 1.74 (m) | 1.82 (m) | 1.99 (m) | 1.34 (m) |
| 11 | 5.14 (dd, 10.3, 5.4) | 5.14 (dd, 10.5, 5.4) | 4.01 (11.0, 5.5) | 3.94 (9.7, 4.9) |
| 12 | 1.16 (ov) | 1.18 (ov) | 1.26 (ov) | 1.19 (ov) |
| 2.31 (dd, 12.2, 5.4) | 2.30 (dd, 12.1, 5.4) | 2.33 (dd, 11.5, 5.5) | 2.34 (11.5, 4.9) | |
| 14 | 1.28 (m) | 1.18 (m) | 1.19 (m) | 1.16 (m) |
| 15 | 1.06 (m), 1.56 (m) | 1.07 (m), 1.79 (m) | 1.09 (m), 1.60 (m) | 1.03 (m), 1.59 (m) |
| 16 | 1.20 (m), 1.88 (m) | 1.26 (m), 1.50 (m) | 1.26 (m), 1.90 (m) | 1.19 (m), 1.78 (m) |
| 17 | 1.15 (m) | 1.18 (m) | 1.19 (m) | 1.19 (m) |
| 18 | 0.73 (s) | 0.75 (s) | 0.66 (s) | 0.73 (s) |
| 19 | 1.28 (s) | 1.28 (s) | 1.21 (s) | 1.32 (s) |
| 20 | 1.38 (m) | 2.00 (m) | 1.37 (m) | 2.30 (m) |
| 21 | 0.90 (d, 6.5) | 0.98 (d, 6.6) | 0.94 (d, 6.5) | 0.92 (d, 6.9) |
| 22 | 1.02 (m), 1.39 (m) | 5.26 (dd, 15.4, 6.2) | 0.89 (m), 1.39 (m) | 4.87 (d, 9.5) |
| 23 | 1.87 (m), 1.99 (m) | 5.20 (dd, 15.4, 7.3) | 0.89 (m), 1.38 (m) | |
| 24 | 5.07 (t, 7.2) | 2.69 (m) | 1.19 (m) | 1.67 (m) |
| 25 | 1.60 (m) | 1.53 (m) | ||
| 26 | 1.59 (s) | 4.70 (br s) | 0.86 (d, 7.0) | 0.84 (d, 6.5) |
| 27 | 1.67 (s) | 1.67 (s) | 0.79 (d, 7.0) | 0.78 (d, 6.5) |
| 28 | 1.07 (d, 6.9) | 0.78 (d, 7.0) | 0.93 (d, 7.1) | |
| 29 | 1.50 (s) | |||
| COO CH3 | 1.98 (s) | 1.98 (s) |
| Position | 5 a | 6 a | 7 a |
|---|---|---|---|
| 1 | 1.43 (m), 1.58 (m) | 1.27 (m), 1.80 (m) | 1.63 (m), 2.16 (m) |
| 2 | 1.55 (m), 1.82 (m) | 1.52 (m), 1.81 (m) | 1.47 (m), 1.85 (m) |
| 3 | 4.11 (m) | 4.06 (m) | 4.05 (m) |
| 4 | 1.63 (ov), 2.09 (ov) | 1.61 (ov), 2.10 (ov) | 1.63 (ov), 2.15 (ov) |
| 6 | 3.59 (br s) | 3.52 (br s) | 3.54 (br s) |
| 7 | 1.50 (m), 1.69 (m) | 1.53 (m), 1.76 (m) | 1.60 (m), 1.74 (m) |
| 8 | 1.72 (m) | 1.84 (m) | 1.85 (m) |
| 9 | 1.40 (m) | 2.08 (m) | 1.62 (m) |
| 11 | 1.40 (m), 1.49 (m) | 5.27 (dd, 11.4, 3.0) | 4.12 (dd, 10.2, 3.0) |
| 12 | 1.51 (m), 2.35 (m) | 3.95 (d, 3.0) | 5.27 (d, 3.0) |
| 14 | 1.74 (m) | 1.88 (m) | 1.72 (m) |
| 15 | 4.64 (d, 5.0) | 1.08 (m), 1.61 (m) | 1.12 (m), 1.63 (m) |
| 16 | 1.65 (m), 1.78 (m) | 1.31 (m), 2.05 (m) | 1.39 (m), 2.00 (m) |
| 17 | 1.96 (m) | 1.68 (m) | |
| 18 | 0.90 (s) | 0.76 (s) | 0.79 (s) |
| 19 | 1.20 (s) | 1.27 (s) | 1.29 (s) |
| 20 | 1.02 (m) | 1.01 (m) | |
| 21 | 1.71 (s) | 1.01 (br s) | 0.93 (d, 6.5) |
| 22 | 1.95(dd, 13.3, 4.3), | 0.23 (ov) | 0.13 (ddd, 9.3, 6.1, 5.7) |
| 2.25 (dd, 12.7, 9.5) | |||
| 23 | 1.80 (m) | ||
| 24 | 1.05 (m) | 0.23 (ov) | 0.24 (m) |
| 25 | 1.62 (m) | 1.50 (m) | 1.63 (m) |
| 26 | 0.83 (d, 6.7) | 0.85 (d, 6.5) | 0.85 (d, 6.9) |
| 27 | 0.89 (d, 8.1) | 0.94 (d, 6.5) | 0.95 (d, 6.5) |
| 28 | 0.79 (d, 6.9) | 0.93 (d, 6.7) | 0.94 (d, 6.4) |
| 29 | 0.70 (d, 6.8) | 0.89 (s) | 0.89 (s) |
| 30 | 0.46 (dd, 8.8, 4.2), | 0.46 (dd, 8.7, 4.4), | |
| −0.14 (d, 5.0) | −0.13 (d, 5.0) | ||
| COO CH3 | 2.08 (s) | 2.17 (s) |
| Compound | Escherichia coli | Bacillus megaterium | Microbotryum violaceum | Septoria tritici | Botrytis cinerea | Chlorella fusca |
|---|---|---|---|---|---|---|
| 1 | 12.0 | 10.0 | 6.5 | 10.5 | 10.5 | 0 |
| 2 | 14.5 | 12.0 | 10.0 | 7.5 | 5.5 | 0 |
| 3 | 7.5 | 4.5 | 7.0 | 10.5 | 5.5 | 0 |
| 4 | 6.0 | 7.0 | 7.5 | 9.0 | 0 | 0 |
| 6 | 9.5 | 10.0 | 8.5 | 10.0 | 8.0 | 0 |
| 7 | 8.0 | 10.0 | 7.0 | 4.5 | 12.0 | 0 |
| 8 | 7.0 | 7.5 | 6.0 | 6.5 | 0 | 0 |
| 9 | 4.5 | 4.5 | 7.0 | 6.5 | 0 | 0 |
| 10 | 6.0 | 8.5 | 8.5 | 4.5 | 0 | 0 |
| 11 | 6.0 | 7.0 | 6.0 | 6.0 | 0 | 0 |
| 12 | 7.0 | 8.0 | 9.5 | 10.0 | 5.0 | 0 |
| 13 | 10.0 | 6.5 | 11.5 | 7.5 | 0 | 0 |
| 14 | 5.0 | 4.0 | 8.5 | 7.0 | 0 | 0 |
| Penicillin | 17.5 | 22.5 | 11.5 | 9.0 | 0 | 0 |
| Strepolin | 8.0 | 7.5 | 6.5 | 9.0 | 0 | 0 |
| Ketoconazole | 10.0 | 10.0 | 20.5 | 20.0 | 26.5 | 0 |
| Acetone | 0 | 0 | 0 | 0 | 0 | 0 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Material and Methods
3.3. Extraction and Isolation
3.4. Agar Diffusion Test for Biological Activity
4. Conclusions
Acknowledgments
References
- Coll, J.C.; Labarre, S.; Sammarco, P.W.; Williams, W.T.; Bakus, G.J. Chemical defenses in soft corals (Coelenterata, Octocorallia) of the great barrier reef: A study of comparative toxicities. Mar. Ecol. Prog. Ser. 1982, 8, 271–278. [Google Scholar] [CrossRef]
- Slattery, M.; McClintock, J.B.; Heine, J.N. Chemical defenses in antarctic soft corals: Evidence for antifouling compounds. J. Exp. Mar. Biol. Ecol. 1995, 190, 61–77. [Google Scholar] [CrossRef]
- Fleury, B.G.; Lages, B.G.; Barbosa, J.P.; Kaiser, C.R.; Pinto, A.C. New hemiketal steroid from the introduced soft coral Chromonephthea braziliensis is a chemical defense against predatory fishes. J. Chem. Ecol. 2008, 34, 987–993. [Google Scholar] [CrossRef]
- Aratake, S.; Tomura, T.; Saitoh, S.; Yokokura, R.; Kawanishi, Y.; Shinjo, R.; Reimer, J.D.; Tanaka, J.; Maekawa, H. Soft coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes. PLoS One 2012, 7, e30410. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, G.V. Chemical constituents of the soft coral species of Sarcophyton genus: A review. J. Indian Chem. Soc. 1997, 74, 272–278. [Google Scholar]
- Zhang, W.; Guo, Y.-W.; Gu, Y.-C. Secondary metabolites from the South China Sea invertebrates: Chemistry and biological activity. Curr. Med. Chem. 2006, 13, 2041–2090. [Google Scholar] [CrossRef]
- Li, C.; La, M.-P.; Li, L.; Li, X.-B.; Tang, H.; Liu, B.-S.; Krohn, K.; Sun, P.; Yi, Y.-H.; Zhang, W. Bioactive 11,20-epoxy-3,5(16)-diene briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. J. Nat. Prod. 2011, 74, 1658–1662. [Google Scholar] [CrossRef]
- Li, C.; La, M.-P.; Sun, P.; Kurtan, T.; Mandi, A.; Tang, H.; Liu, B.-S.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive (3Z,5E)-11,20-epoxybriara-3,5-dien-7,18-olide diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs 2011, 9, 1403–1418. [Google Scholar] [CrossRef]
- Li, C.; La, M.-P.; Tang, H.; Pan, W.-H.; Sun, P.; Krohn, K.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012, 22, 4368–4372. [Google Scholar]
- Geng, W.-L.; Wang, X.-Y.; Kurtán, T.; Mándi, A.; Tang, H.; Schulz, B.; Sun, P.; Zhang, W. Herbarone, a rearranged heptaketide derivative from the sea hare associated fungus Torula herbarum. J. Nat. Prod. 2012, 75, 1828–1832. [Google Scholar] [CrossRef]
- Sun, P.; Meng, L.-Y.; Tang, H.; Liu, B.-S.; Li, L.; Yi, Y.-H.; Zhang, W. Sinularosides A and B, bioactive 9,11-secosteroidal glycosides from the South China Sea soft coral Sinularia humilis Ofwegen. J. Nat. Prod. 2012, 75, 1656–1659. [Google Scholar] [CrossRef]
- Edrada, R.A.; Wray, V.; Witte, L.; van Ofwegen, L.P.; Proksch, P. Bioactive terpenes from the soft coral Heteroxenia sp. from Mindoro, Philippines. Z. Naturforsch. C 2000, 55, 82–86. [Google Scholar]
- Umeyama, A.; Shoji, N.; Ozeki, M.; Arihara, S. Sarcoaldesterols A and B, two new polyhydroxylated sterols from the soft coral Sarcophyton sp. J. Nat. Prod. 1996, 59, 894–895. [Google Scholar] [CrossRef]
- Mohammed, R.; Seliem, M.A.E.; Mohammed, T.A.A.; Abed-El Fatah, A.; Abo-Youssef, A.M.; Thabet, M.M. Bioactive secondary metabolites from the Red Sea soft coral Heteroxenia fuscescens. Int. J. Appl. Res. Nat. Prod. 2012, 4, 15–27. [Google Scholar]
- Rao, C.B.; Ramana, K.V.; Rao, D.V.; Fahy, E.; Faulkner, D.J. Metabolites of the gorgonian Isis hippuris from India. J. Nat. Prod. 1988, 51, 954–958. [Google Scholar] [CrossRef]
- Lu, Q.; Faulkner, D.J. Two 11α-acetoxysterols from the Palauan soft coral Lobophytum cf. pauciflorum. Nat. Prod. Lett. 1997, 10, 231–237. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abdel-Razik, A.F.; Nassar, M.I.; Mohamed, T.K.; Ibrahim, M.A.; El-Kousy, S.M. A new gorgostane derivative from the Egyptian Red Sea soft coral Heteroxenia ghardaqensis. Nat. Prod. Res. 2012. [Google Scholar] [CrossRef]
- Van Minh, C.; Cuong, N.X.; Tuan, T.A.; Choi, E.M.; Kim, Y.H.; van Kiem, P. A new 9,11-secosterol from the Vietnamese sea soft coral, Sarcophyton mililatensis, increases the function of osteoblastic MC3T3-E1 cells. Nat. Prod. Commun. 2007, 2, 1095–1100. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Xiao, Z.-H.; Huang, J.-S.; Wu, J.; Li, Q.-X. Study on the chemical constituents of the South China Sea gorgonian Junceella juncea. Chem. Pharm. Bull. 2004, 52, 1476–1478. [Google Scholar] [CrossRef]
- Rueda, A.; Zubia, E.; Ortega, M.J.; Salva, J. Structure and cytotoxicity of new polyhydroxylated sterols from the Caribbean gorgonian Plexaurella grisea. Steroids 2001, 66, 897–904. [Google Scholar] [CrossRef]
- Duh, C.Y.; Wang, S.-K.; Chung, S.G.; Chou, G.-C.; Dai, C.-F. Cytotoxic cembrenolides and steroids from the formosan soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Iorizzi, M.; Minale, L.; Riccio, R.; Lee, J.S.; Yasumoto, T. Polar steroids from the marine scallop Patinopecten yessoensis. J. Nat. Prod. 1988, 51, 1098–1103. [Google Scholar] [CrossRef]
- Madaio, A.; Piccialli, V.; Sica, D.; Corriero, G. New polyhydroxysterols from the dictyoceratid sponges Hippospongia-communis, Spongia-officinalis, Ircinia-variabilis, and Spongionella-gracilis. J. Nat. Prod. 1989, 52, 952–961. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M.; Carnuccio, R.; Iuvone, T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the Lagoon of Venice. Steroids 1995, 60, 666–673. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.W.; Mollo, E.; Fontana, A.; Cimino, G. Acanthovagasteroids A-D, four new 19-hydroxylated steroids from the South China Sea Gorgonian Acanthogorgia vagae aurivillius. J. Nat. Prod. 2004, 67, 2083–2085. [Google Scholar] [CrossRef]
- Piccialli, V.; Sica, D. New dihydroxylated sterols from the marine sponge Spongionella gracilis. J. Nat. Prod. 1986, 49, 779–783. [Google Scholar] [CrossRef]
- Nakatsu, T.; Walker, R.P.; Thompson, J.E.; Faulkner, D.J. Biologically-active sterol sulfates from the marine sponge Toxadocia zumi. Experientia 1983, 39, 759–761. [Google Scholar] [CrossRef]
- Akihisa, T.; Ghosh, P.; Thakur, S.; Oshikiri, S.; Tamura, T.; Matsumoto, T. 24-β-methyl cholesta-5,22E,25-trien-3-β-ol and 24-α-ethyl-5-α-cholest-22E-en-3-β-ol from Clerodendrum fragrans. Phytochemistry 1988, 27, 241–244. [Google Scholar]
- Shimizu, Y.; Alam, M.; Kobayashi, A. Letter: Dinosterol, the major sterol with a unique side chain ion the toxic dinoflagellate, Gonyaulax tamarensis. J. Am. Chem. Soc. 1976, 98, 1059–1060. [Google Scholar] [CrossRef]
- Finer, J.; Clardy, J.; Kobayashi, A.; Alam, M.; Shimizu, Y. Identity of stereochemistry of dinosterol and gorgosterol side chain. J. Org. Chem. 1978, 43, 1990–1992. [Google Scholar] [CrossRef]
- Shu, A.Y.L.; Djerassi, C. Stereospecific synthesis of dinosterol. Tetrahedron Lett. 1981, 22, 4627–4630. [Google Scholar] [CrossRef]
- Giner, J.L.; Djerassi, C. Biosynthetic studies of marine lipids. 33. Biosynthesis of dinosterol, peridinosterol, and gorgosterol: Unusual patterns of bioalkylation in dinoflagellate sterols. J. Org. Chem. 1991, 56, 2357–2363. [Google Scholar] [CrossRef]
- Rao, M.R.; Venkatesham, U.; Reddy, M.V.R.; Venkateswarlu, Y. An unusual novel C-29 steroid from the soft coral Lobophytum crassum. J. Nat. Prod. 1999, 62, 785–786. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Rivera, J.; Boulanger, A. New polyhydroxydinostane sterols from the Caribbean gorgonian octocoral Pseudopterogorgia americana. Tetrahedron Lett. 1998, 39, 7645–7648. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomioka, A.; Mitsuhashi, H. Marine sterols. 8. Isolation and structure of sarcosterol, a new sterol with a ∆17(20) double bond from the soft coral sarcophyton glaucum. Steroids 1979, 34, 273–284. [Google Scholar] [CrossRef]
- Kobayashi, M. Marine sterols. 27. 25-hydroxy derivative of sarcosterol, a novel marine sterol with a 23-methyl and a 17(20)E-double bond, from the soft coral Sinularia mayi. Steroids 1994, 59, 27–29. [Google Scholar] [CrossRef]
- Swenson, W.; Tagle, B.; Clardy, J.; Withers, N.W.; Kokke, W.; Fenical, W.; Djerassi, C. Peridinosterol—a new ∆17 unsaturated sterol from two cultured marine algae. Tetrahedron Lett. 1980, 21, 4663–4666. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Ullah, Z.; Florke, U.; Pescitelli, G.; Di Bari, L.; Antus, S.; Kurtan, T.; Rheinheimer, J.; Draeger, S.; Schulz, B. New mono- and dimeric members of the secalonic acid family: Blennolides A–G isolated from the fungus Blennoria sp. Chem. Eur. J. 2008, 14, 4913–4923. [Google Scholar] [CrossRef]
- Ulrich Höller, U.; Wright, A.D.; Matthee, G.F.; Konig, G.M.; Draeger, S.; Aust, H.J.; Schulz, B. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycol.Res. 2000, 104, 1354–1365. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775-787. https://doi.org/10.3390/md11030775
Wang Z, Tang H, Wang P, Gong W, Xue M, Zhang H, Liu T, Liu B, Yi Y, Zhang W. Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp. Marine Drugs. 2013; 11(3):775-787. https://doi.org/10.3390/md11030775
Chicago/Turabian StyleWang, Zenglei, Hua Tang, Pan Wang, Wei Gong, Mei Xue, Hongwei Zhang, Taofang Liu, Baoshu Liu, Yanghua Yi, and Wen Zhang. 2013. "Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp." Marine Drugs 11, no. 3: 775-787. https://doi.org/10.3390/md11030775
APA StyleWang, Z., Tang, H., Wang, P., Gong, W., Xue, M., Zhang, H., Liu, T., Liu, B., Yi, Y., & Zhang, W. (2013). Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp. Marine Drugs, 11(3), 775-787. https://doi.org/10.3390/md11030775