The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis
Abstract
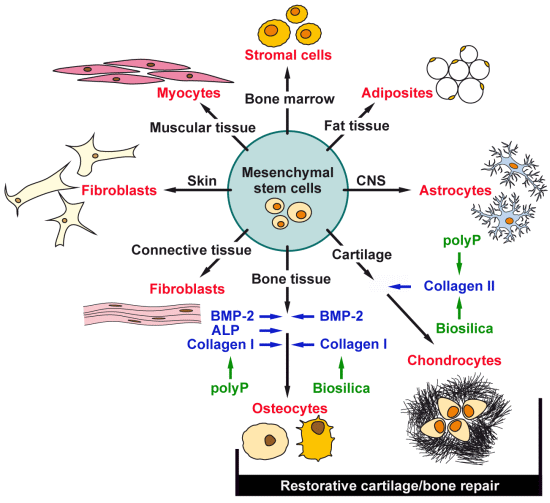
:1. Introduction

2. Results and Discussion
2.1. Cultivation of hMSCs and Encapsulation into Alginate Beads

2.2. Mineralization of the Cells, Triggered to Osteogenic Differentiation

2.3. Osteogenic versus Chondrogenic Differentiation: Effect of Biosilica and Polyp on BMP-2 Expression

2.4. Osteogenic versus Chondrogenic Differentiation: Expression of ALP

2.5. Osteogenic versus Chondrogenic Differentiation: Expression of Collagen Type I

2.6. Osteogenic versus Chondrogenic Differentiation: Expression of the Collagen Type II

2.7. Discussion

3. Experimental Section
3.1. Isolation and Cultivation of Human MSCs
3.2. Preparation of Alginate/Silica and Alginate/PolyP (Ca2+ Salt) Composite Hydrogel Beads
3.3. Differentiation Assays in Vitro
3.4. Mineralization Assay with Alizarin Red S
3.5. Quantitative Real-Time RT-PCR (qRT-PCR) Analysis
3.6. Further Analyses
4. Conclusion
Acknowledgments
Conflicts of Interest
References
- Parra-Torres, A.Y.; Valdés-Flores, M.; Orozco, L.; Velázquez-Cruz, R. Molecular Aspects of Bone Remodeling. In Topics in Osteoporosis; InTech: Rijeka, Croatia, 2013; pp. 1–27. [Google Scholar]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Beyth, S.; Schröder, J.; Liebergall, M. Stem cells in bone diseases: Current clinical practice. Br. Med. Bull. 2011, 99, 199–210. [Google Scholar] [CrossRef]
- Sunay, O.; Can, G.; Cakir, Z.; Denek, Z.; Kozanoglu, I.; Erbil, G.; Yilmaz, M.; Baran, Y. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy 2013, 15, 690–702. [Google Scholar] [CrossRef]
- Panetta, N.J.; Gupta, D.M.; Slater, B.J.; Kwan, M.D.; Liu, K.J.; Longaker, M.T. Tissue engineering in cleft palate and other congenital malformations. Pediatr. Res. 2008, 63, 545–551. [Google Scholar] [CrossRef]
- Gessmann, J.; Köller, M.; Godry, H.; Schildhauer, T.A.; Seybold, D. Regenerate augmentation with bone marrow concentrate after traumatic bone loss. Orthop. Rev. 2012, 4, e14. [Google Scholar]
- Müller, W.E.G.; Schröder, H.C.; Feng, Q.L.; Schloßmacher, U.; Link, T.; Wang, X.H. Development of a morphogenetically active scaffold for three-dimensional growth of bone cells: Biosilica/Alginate hydrogel for SaOS-2 cell cultivation. J. Tissue Engin. Regener. Med. 2013. [Google Scholar] [CrossRef]
- Ilizarov, G.A. Clinical application of the tension–stress effect for limblengthening. Clin. Orthop. Relat. Res. 1990, 250, 8–26. [Google Scholar]
- Cope, J.B.; Samchukov, M.L.; Cherkashin, A.M. Mandibular distraction osteogenesis: A historic perspective and future directions. Am. J. Orthod. Dentofacial Orthop. 1999, 115, 448–460. [Google Scholar] [CrossRef]
- Jazrawi, L.M.; Majeska, R.J.; Klein, M.L.; Kagel, E.; Stromberg, L.; Einhorn, T.A. Bone and cartilage formation in an experimental model of distraction osteogenesis. J. Orthop. Trauma 1998, 12, 111–116. [Google Scholar] [CrossRef]
- Sato, M.; Yasui, N.; Nakase, T.; Kawahata, H.; Sugimoto, M.; Hirota, S.; Kitamura, Y.; Nomura, S.; Ochi, T. Expression of bone matrix proteins mRNA during distraction osteogenesis. J. Bone Miner. Res. 1998, 13, 1221–1231. [Google Scholar] [CrossRef]
- Matsubara, H.; Hogan, D.E.; Morgan, E.F.; Mortlock, D.P.; Einhorn, T.A.; Gerstenfeld, L.C. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 2012, 51, 168–180. [Google Scholar] [CrossRef]
- Hegab, A.F.; Shuman, M.A. Distraction osteogenesis of the maxillofacial skeleton: biomechanics and clinical implications. Sci. Rep. 2012, 1, 1–12. [Google Scholar]
- Müller, W.E.G.; Wang, X.H.; Grebenjuk, V.; Diehl-Seifert, B.; Steffen, R.; Schloßmacher, U.; Trautwein, A.; Neumann, S.; Schröder, H.C. Silica as a morphogenetically active inorganic polymer: effect on the BMP-2-dependent and RUNX2-independent pathway in osteoblast-like SaOS-2 cells. Biomater. Sci. 2013, 1, 669–678. [Google Scholar] [CrossRef]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. 2012, 37, 227–258. [Google Scholar]
- Wang, X.H.; Schröder, H.C.; Wiens, M.; Ushijima, H.; Müller, W.E.G. Bio-silica and bio-polyphosphate: Applications in biomedicine (bone formation). Curr. Opin. Biotechnol. 2012, 23, 570–578. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Burghard, Z.; Pisignano, D.; Wang, X.H. Silicateins—A novel paradigm in bioinorganic chemistry: Enzymatic synthesis of inorganic polymeric silica. Chem. Eur. J. 2013, 19, 5790–5804. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar]
- Schwarz, K.; Milne, D.B. Growth-promoting effects of silicon in rats. Nature 1972, 239, 333–334. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.H.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E.G. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar]
- Wiens, M.; Wang, X.H.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Ushijima, H.; Schröder, H.C.; Müller, W.E.G. Osteogenic potential of bio-silica on human osteoblast-like (SaOS-2) cells. Calcif. Tissue Int. 2010, 87, 513–524. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Albert, O.; Schröder, H.C.; Wang, X.H. Bio-inorganic nanomaterials for biomedical applications (Bio-silica and polyphosphate). In Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S., Sigmund, W., Zauscher, S., Eds.; Springer-Press: Berlin, Germany, 2014; in press. [Google Scholar]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–50. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Deshmukh, K.; Sawyer, B.D. Synthesis of collagen by chondrocytes in suspension culture: modulation by calcium, 3′:5′-cyclic AMP, and prostaglandins. Proc. Natl. Acad. Sci. USA 1977, 74, 3864–3868. [Google Scholar] [CrossRef]
- Schlossmacher, U.; Wiens, M.; Schröder, H.C.; Wang, X.H.; Jochum, K.P.; Müller, W.E.G. Silintaphin-1: Interaction with silicatein during structureguiding biosilica formation. FEBS J. 2011, 278, 1145–1155. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef]
- Xu, Y.; Pritzker, K.P.; Cruz, T.F. Characterization of chondrocyte alkaline phosphatase as a potential mediator in the dissolution of calcium pyrophosphate dihydrate crystals. J. Rheumatol. 1994, 21, 912–919. [Google Scholar]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.; Evans, B.A.; Thompson, R.P.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Ma, H.L.; Hung, S.C.; Lin, S.Y.; Chen, Y.L.; Lo, W.H. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J. Biomed. Mater. Res. A 2003, 64, 273–281. [Google Scholar]
- Schloßmacher, U.; Schröder, H.C.; Wang, X.H.; Feng, Q.; Diehl-Seifert, B.; Neumann, S.; Trautwein, A.; Müller, W.E.G. Alginate/silica composite hydrogel as a potential morphogenetically active scaffold for three-dimensional tissue engineering. RSC Adv. 2013, 3, 11185–11194. [Google Scholar] [CrossRef]
- Lim, H.J.; Ghim, H.D.; Choi, J.H.; Chung, H.Y.; Lim, J.O. Controlled release of BMP-2 from alginate nanohydrogels enhanced osteogenic differentiation of human bone marrow stromal cells. Macromol. Res. 2010, 18, 787–792. [Google Scholar] [CrossRef]
- Wang, X.H.; Schröder, H.C.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Wiens, M.; Müller, W.E.G. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J. Tissue Eng. Regen. Med. 2013, 7, 767–776. [Google Scholar]
- Mandu-Hrit, M.; Haque, T.; Lauzier, D.; Kotsiopriftis, M.; Rauch, F.; Tabrizian, M.; Henderson, J.E.; Hamdy, R.C. Early injection of OP-1 during distraction osteogenesis accelerates new bone formation in rabbits. Growth Factors 2006, 24, 172–183. [Google Scholar] [CrossRef]
- Osyczka, A.M.; Diefenderfer, D.L.; Bhargave, G.; Leboy, P.S. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs 2004, 176, 109–119. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Bianco, P.; Vetter, U.; Robey, P.G. Human bone cell enzyme expression and cellular heterogeneity: Correlation of alkaline phosphatase enzyme activity with cell cycle. J. Cell Physiol. 1990, 144, 115–121. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Shi, S.; Kirk, M.; Kahn, A.J. The role of type I collagen in the regulation of the osteoblast phenotype. J. Bone Miner. Res. 1996, 11, 1139–1145. [Google Scholar]
- Chen, C.W.; Tsai, Y.H.; Deng, W.P.; Shih, S.N.; Fang, C.L.; Burch, J.G.; Chen, W.H.; Lai, W.F. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J. Orthop. Res. 2005, 23, 446–453. [Google Scholar]
- Lorenz, M.R.; Holzapfel, V.; Musyanovych, A.; Nothelfer, K.; Walther, P.; Frank, H.; Landfester, K.; Schrezenmeier, H.; Mailänder, V. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials 2006, 27, 2820–2828. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Schröder, H.C.; Borejko, A.; Krasko, A.; Reiber, A.; Schwertner, H.; Müller, W.E.G. Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J. Biomed. Mat. Res. B 2005, 75B, 387–392. [Google Scholar] [CrossRef]
- Sachs, L. Angewandte Statistik; Springer: Berlin, Germany, 1984; p. 242. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Schröder, H.C.; Grebenjuk, V.; Diehl-Seifert, B.; Mailänder, V.; Steffen, R.; Schloßmacher, U.; Müller, W.E.G. The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis. Mar. Drugs 2014, 12, 1131-1147. https://doi.org/10.3390/md12021131
Wang X, Schröder HC, Grebenjuk V, Diehl-Seifert B, Mailänder V, Steffen R, Schloßmacher U, Müller WEG. The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis. Marine Drugs. 2014; 12(2):1131-1147. https://doi.org/10.3390/md12021131
Chicago/Turabian StyleWang, Xiaohong, Heinz C. Schröder, Vladislav Grebenjuk, Bärbel Diehl-Seifert, Volker Mailänder, Renate Steffen, Ute Schloßmacher, and Werner E. G. Müller. 2014. "The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis" Marine Drugs 12, no. 2: 1131-1147. https://doi.org/10.3390/md12021131
APA StyleWang, X., Schröder, H. C., Grebenjuk, V., Diehl-Seifert, B., Mailänder, V., Steffen, R., Schloßmacher, U., & Müller, W. E. G. (2014). The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis. Marine Drugs, 12(2), 1131-1147. https://doi.org/10.3390/md12021131