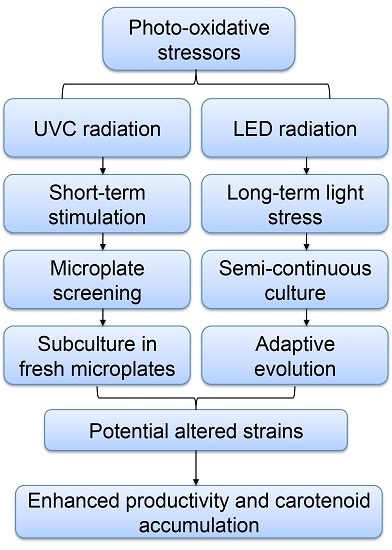
Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the Marine Diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation
Abstract
:1. Introduction

2. Results and Discussion
2.1. Screening of P. tricornutum Mutants

2.2. UVC Treatment Induced Accumulation of Neutral Lipids

2.3. Carotenoids and Chlorophyll a Contents in Wild Type P. tricornutum and its UV-Mutants

2.4. Adaptive Laboratory Evolution (ALE) Increased Growth Rate and Fucoxanthin Content


3. Experimental Design and Methods
3.1. Diatom Culture and Growth Conditions
3.2. UV Mutagenesis
3.3. Growth Measurements and Calculations
3.4. Gravimetric Method for Determining Total Neutral Lipid Content
3.5. Nile Red Staining for Neutral Lipid Detection
3.6. Adaptive Laboratory Evolution (ALE)
3.7. Artificial Light Setup
3.8. Chlorophyll and Carotenoid Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, N.; Dries, R.; Burson, A.; Stal, L.J.; Sloot, P.M.; Kaandorp, J.A. Temperature affects the silicate morphology in a diatom. Sci. Rep. 2015, 5, 11652. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Bartual, A.; Willis, A.; Meichenin, A.; Villazan, B.; Maheswari, U.; Bowler, C. Physiological and molecular evidence that environmental changes elicit morphological interconversion in the model diatom Phaeodactylum tricornutum. Protist 2011, 162, 462–481. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.K.; Niu, Y.F.; Ma, Y.H.; Xue, J.; Zhang, M.H.; Yang, W.D.; Liu, J.S.; Lu, S.H.; Guan, Y.; Li, H.Y. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation. Biotechnol. Biofuels 2013, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Alternat. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Hlavova, M.; Turoczy, Z.; Bisova, K. Improving microalgae for biotechnology—From genetics to synthetic biology. Biotechnol. Adv. 2015, 33. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.Y.; Yang, Z.K.; Zheng, J.W.; Xie, Y.; Li, D.W.; Murugan, S.B.; Yang, W.D.; Liu, J.S.; Li, H.Y. Examination of metabolic responses to phosphorus limitation via proteomic analyses in the marine diatom Phaeodactylum tricornutum. Sci. Rep. 2015, 5, 10373. [Google Scholar] [CrossRef] [PubMed]
- Daboussi, F.; Leduc, S.; Marechal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Binti Ibnu Rasid, E.N.; Mohamad, S.E.; Jamaluddin, H.; Salleh, M.M. Screening factors influencing the production of astaxanthin from freshwater and marine microalgae. Appl. Biochem. Biotechnol. 2014, 172, 2160–2174. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Paglia, G.; Magnusdottir, M.; Steinarsdottir, E.A.; Gudmundsson, S.; Palsson, B.O.; Andresson, O.S.; Brynjolfsson, S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Fact. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Wichuk, K.; Brynjolfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208. [Google Scholar] [PubMed]
- Sandesh Kamath, B.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tillich, U.M.; Lehmann, S.; Schulze, K.; Duhring, U.; Frohme, M. The optimal mutagen dosage to induce point-mutations in Synechocystis sp. PCC6803 and its application to promote temperature tolerance. PloS ONE 2012, 7, e49467. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution-principles and applications for biotechnology. Microb. Cell Fact. 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Guethmundsson, O.; Paglia, G.; Herjolfsson, G.; Andresson, O.S.; Palsson, B.O.; Brynjolfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Gudmundsson, O.; Feist, A.M.; Herjolfsson, G.; Brynjolfsson, S.; Palsson, B.O. Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. J. Biotechnol. 2012, 161, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Meireles, L.A.; Guedes, A.C.; Malcata, F.X. Increase of the yields of eicosapentaenoic and docosahexaenoic acids by the microalga Pavlova lutheri following random mutagenesis. Biotechnol. Bioeng. 2003, 81, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, S.; van Graff Creveld, S.; Schatz, D.; Malitsky, S.; Tzfadia, O.; Aharoni, A.; Levin, Y.; Gabashvili, A.; Feldmesser, E.; Vardi, A. Mapping the diatom redox-sensitive proteome provides insight into response to nitrogen stress in the marine environment. Proc. Natl. Acad. Sci. USA 2014, 111, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Lukavsky, J.; Nedbalova, L.; Kolouchova, I.; Sigler, K. Effect of starvation on the distribution of positional isomers and enantiomers of triacylglycerol in the diatom Phaeodactylum tricornutum. Phytochemistry 2012, 80, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Ahmed, F.; Schenk, P.M.; Li, Y. UV-C mediated rapid carotenoid induction and settling performance of Dunaliella salina and Haematococcus pluvialis. Biotechnol. Bioeng. 2015, 112, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Pacini, T.; Fu, W.; Gudmundsson, S.; Chiaravalle, A.E.; Brynjolfson, S.; Palsson, B.O.; Astarita, G.; Paglia, G. Multidimensional analytical approach based on UHPLC-UV-ion mobility-MS for the screening of natural pigments. Anal. Chem. 2015, 87, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wichuk, K.; Brynjolfsson, S. Developing diatoms for value-added products: Challenges and opportunities. N. Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Magnusdottir, M.; Brynjolfson, S.; Palsson, B.O.; Paglia, G. UPLC-UV-MS(E) analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Xu, M.; Magnusdottir, M.; Zhang, Y.; Brynjolfsson, S.; Fu, W. Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the Marine Diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation. Mar. Drugs 2015, 13, 6138-6151. https://doi.org/10.3390/md13106138
Yi Z, Xu M, Magnusdottir M, Zhang Y, Brynjolfsson S, Fu W. Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the Marine Diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation. Marine Drugs. 2015; 13(10):6138-6151. https://doi.org/10.3390/md13106138
Chicago/Turabian StyleYi, Zhiqian, Maonian Xu, Manuela Magnusdottir, Yuetuan Zhang, Sigurdur Brynjolfsson, and Weiqi Fu. 2015. "Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the Marine Diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation" Marine Drugs 13, no. 10: 6138-6151. https://doi.org/10.3390/md13106138
APA StyleYi, Z., Xu, M., Magnusdottir, M., Zhang, Y., Brynjolfsson, S., & Fu, W. (2015). Photo-Oxidative Stress-Driven Mutagenesis and Adaptive Evolution on the Marine Diatom Phaeodactylum tricornutum for Enhanced Carotenoid Accumulation. Marine Drugs, 13(10), 6138-6151. https://doi.org/10.3390/md13106138