A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction
Abstract
:1. Introduction
2. Experimental Design
3. Results and Discussion
3.1. Trueness and Precision
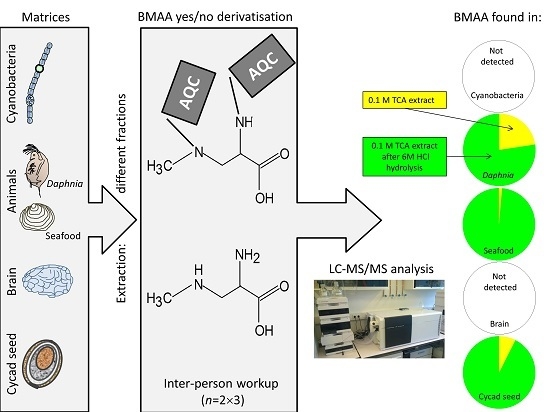
3.2. BMAA in Blanks and Cycad Samples
3.3. BMAA in Brain Tissue
3.4. BMAA in Animal and Cyanobacterial Samples
3.5. BMAA Fractions
4. Conclusions and Outlook
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam amyotrophic lateral sclerosis-Parkinsonism-dementia linked to a plant excitant neurotoxin. Science 1987, 237, 517–522. [Google Scholar] [CrossRef]
- Bradley, W.G.; Mash, D.C. Beyond Guam: The cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 7–20. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J. Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins 2014, 6, 1109–1138. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Gillissen, F.; Zweers, H.A.J.; Lürling, M. Determination of the neurotoxins BMAA (β-N-methylamino-l-alanine) and DAB (α-,γ-diaminobutyric acid) by LC-MSMS in Dutch urban waters with cyanobacterial blooms. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spáčil, Z.; Ilag, L.L.; Ronnevi, L.O.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; García-Altares, M.; Mendes e Mello, M.; Lürling, M. Trans generational effects of the neurotoxin BMAA on the aquatic grazer Daphnia magna. Aquat. Toxicol. 2015, 168, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Faassen, E.J.; Eenennaam, J.S.V. Effects of the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) on the survival, mobility and reproduction of Daphnia magna. J. Plankton Res. 2011, 33, 333–342. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Wiegand, C.; Downing, T.G. β-N-methylamino-l-alanine (BMAA) uptake by the animal model, Daphnia magna and subsequent oxidative stress. Toxicon 2015, 100, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Contardo-Jara, V.; Pflugmacher, S.; Downing, T.G. The fate of the cyanobacterial toxin β-N-methylamino-l-alanine in freshwater mussels. Ecotoxicol. Environ. Saf. 2014, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, M.; Pflugmacher, S.; Downing, T.G. β-N-Methylamino-l-alanine (BMAA) uptake by the aquatic macrophyte Ceratophyllum demersum. Ecotoxicol. Environ. Saf. 2011, 74, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Lampinen Salomonsson, M.; Hansson, A.; Bondesson, U. Development and in-house validation of a method for quantification of BMAA in mussels using dansyl chloride derivatization and ultra performance liquid chromatography tandem mass spectrometry. Anal. Methods 2013, 5, 4865–4874. [Google Scholar] [CrossRef]
- Réveillon, D.; Abadie, E.; Séchet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-methylamino-l-alanine: LC-MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean Lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kiselova, N.; Rosén, J.; Ilag, L.L. Quantification of neurotoxin BMAA (β-N-methylamino-l-alanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS ONE 2012, 7, e36667. [Google Scholar] [CrossRef] [PubMed]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.W. Good mass spectrometry and its place in good science. J. Mass Spectrom. 2012, 47, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A. Analytical techniques for the detection of α-amino-β-methylaminopropionic acid. Analyst 2012, 137, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Rosén, J.; Westerberg, E.; Schmiedt, S.; Hellenäs, K.E. BMAA detected as neither free nor protein bound amino acid in blue mussels. Toxicon 2016, 109, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rodgers, K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of l-serine causing protein misfolding and aggregation. PLoS ONE 2013, 8, e75376. [Google Scholar] [CrossRef] [PubMed]
- Glover, W.B.; Mash, D.C.; Murch, S.J. The natural non-protein amino acid N-β-methylamino-l-alanine (BMAA) is incorporated into protein during synthesis. Amino Acids 2014, 46, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Van Onselen, R.; Cook, N.A.; Phelan, R.R.; Downing, T.G. Bacteria do not incorporate β-N-methylamino-l-alanine into their proteins. Toxicon 2015, 102, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Banack, S.A. Previous studies underestimate BMAA concentrations in cycad flour. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 41–43. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Abadie, E.; Séchet, V.; Masseret, E.; Hess, P.; Amzil, Z. β-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar. Environ. Res. 2015, 110, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, M.; Downing, T.G. β-N-methylamino-l-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Esterhuizen-Londt, M.; Grant Downing, T. β-N-methylamino-l-alanine (BMAA) metabolism in the aquatic macrophyte Ceratophyllum demersum. Ecotoxicol. Environ. Saf. 2015, 120, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Rosén, J.; Hellenäs, K.E. Determination of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC-MS/MS (liquid chromatography tandem mass spectrometry). Analyst 2008, 133, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Fan, H.; Ma, F.; McCarron, P.; Thomas, K.; Tang, X.; Quilliam, M.A. Elucidation of matrix effects and performance of solid-phase extraction for LC-MS/MS analysis of β-N-methylamino-l-alanine (BMAA) and 2,4-diaminobutyric acid (DAB) neurotoxins in cyanobacteria. Analyst 2012, 137, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; El Abdellaoui, S.; Sarazin, C.; Vial, J.; Mejean, A.; Ploux, O.; Pichon, V. Validation of the analytical procedure for the determination of the neurotoxin β-N-methylamino-l-alanine in complex environmental samples. Anal. Chim. Acta 2013, 771, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Aigret, B.; De Borggraeve, W.M.; Spacil, Z.; Ilag, L.L. Selective LC-MS/MS method for the identification of BMAA from its isomers in biological samples. Anal. Bioanal. Chem. 2012, 403, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-l-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Solliec, M.; Sauvé, S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5487–5501. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; El Abdellaoui, S.; Vial, J.; Lagrange, E.; Pichon, V. Development of an analytical procedure for quantifying the underivatized neurotoxin β-N-methylamino-l-alanine in brain tissues. Anal. Bioanal. Chem. 2014, 406, 4627–4636. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Burian, A.; Rasmussen, U.; Costa, P.R.; Annadotter, H.; Godhe, A.; Rydberg, S. BMAA extraction of cyanobacteria samples: Which method to choose? Environ. Sci. Pollut. Res. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: http://ec.europa.eu/food/plant/pesticides/guidance_documents/docs/qualcontrol_en.pdf (accessed on 24 August 2015).
- Beach, D.G.; Kerrin, E.S.; Quilliam, M.A. Selective quantitation of the neurotoxin BMAA by use of hydrophilic-interaction liquid chromatography-differential mobility spectrometry-tandem mass spectrometry (HILIC–DMS–MS/MS). Anal. Bioanal. Chem. 2015, 407, 8379–8409. [Google Scholar] [CrossRef] [PubMed]





| Protocol | Animal (A) | Brain (B) | Cyanobacteria (C) | ||||
|---|---|---|---|---|---|---|---|
| Fraction | Free | T.S. 1 | Total | Free | Total | Free | Total |
| Blank | 85 (2.6) | (4.9) | 81 (13.7) | 78 (4.8) | 72 (8.4) | 100 (7.8) | (6.3) |
| Cycad | 93 (7.8) | (11.4) | 86 (2.1) * | (7.5) | 73 (2.5) | 103 (8.5) | (4.3) |
| Seafood | 96 (6.6) | 78 (7.9) | 108 (6.7) | - | - | - | - |
| Daphnia magna | (2.5) | 75 (1.0) | 110 (8.0) | - | - | - | - |
| Brain unspiked | - | - | - | 77 (11.1) | 84 (15.7) | - | - |
| Brain spiked | - | - | - | 80 (6.0) | 82 (9.0) | - | - |
| Anabaena | - | - | - | - | - | 103 (7.4) | 78 (2.3) |
| Leptolyngbya | - | - | - | - | - | ||
| Protocol | Animal (A) | Brain (B) | Cyanobacteria (C) | ||||
|---|---|---|---|---|---|---|---|
| Fraction | Free | T. S. 1 | Total | Free | Total | Free | Total |
| uncorrected for D3BMAA | 10.3 | 8.4 | * | 13.5 | 18.5 | ||
| corrected for D3BMAA | 10.4 | 13.6 | * | 9.2 | 11.6 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faassen, E.J.; Antoniou, M.G.; Beekman-Lukassen, W.; Blahova, L.; Chernova, E.; Christophoridis, C.; Combes, A.; Edwards, C.; Fastner, J.; Harmsen, J.; et al. A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction. Mar. Drugs 2016, 14, 45. https://doi.org/10.3390/md14030045
Faassen EJ, Antoniou MG, Beekman-Lukassen W, Blahova L, Chernova E, Christophoridis C, Combes A, Edwards C, Fastner J, Harmsen J, et al. A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction. Marine Drugs. 2016; 14(3):45. https://doi.org/10.3390/md14030045
Chicago/Turabian StyleFaassen, Elisabeth J., Maria G. Antoniou, Wendy Beekman-Lukassen, Lucie Blahova, Ekaterina Chernova, Christophoros Christophoridis, Audrey Combes, Christine Edwards, Jutta Fastner, Joop Harmsen, and et al. 2016. "A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction" Marine Drugs 14, no. 3: 45. https://doi.org/10.3390/md14030045
APA StyleFaassen, E. J., Antoniou, M. G., Beekman-Lukassen, W., Blahova, L., Chernova, E., Christophoridis, C., Combes, A., Edwards, C., Fastner, J., Harmsen, J., Hiskia, A., Ilag, L. L., Kaloudis, T., Lopicic, S., Lürling, M., Mazur-Marzec, H., Meriluoto, J., Porojan, C., Viner-Mozzini, Y., & Zguna, N. (2016). A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction. Marine Drugs, 14(3), 45. https://doi.org/10.3390/md14030045