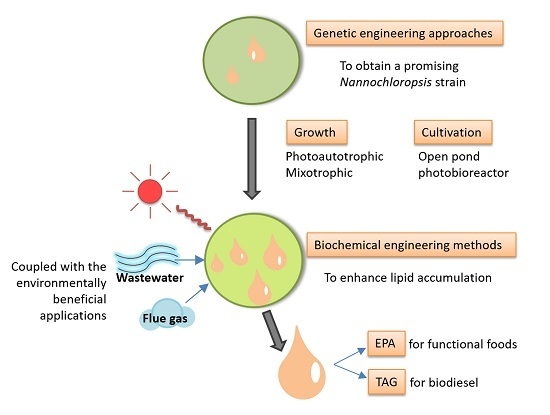
Lipid Production from Nannochloropsis
Abstract
:1. Introduction
2. Taxonomy and Morphology of Nannochloropsis
3. Nannochloropsis as a Promising Cell Factory for Lipid Production
3.1. Cell Growth and Lipid Accumulation
3.2. Fatty Acid Profile
3.3. EPA Production
3.4. Waste Water Bioremediation and CO2 Biomitigation
4. Cultivation of Nannochloropsis
4.1. Photoautotrophic Growth
4.2. Heterotrophic Growth
4.3. Mixotrophic Growth
5. Biochemical Engineering Methods Enhancing Lipid Accumulation in Nannochloropsis
5.1. Irradiance
5.2. Nitrogen
5.3. Salinity
5.4. Combined Stress Factors
6. Lipid Synthesis Pathway
6.1. The Physiological Role of Lipids
6.2. De Novo Fatty Acid Synthesis
6.3. TAG Formation
7. Genetic Engineering Approaches for Lipid Production in Nannochloropsis
8. Future Prospects of Nannochloropsis
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-H.; Chen, F.; Wei, D.; Zhang, X.-W.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.-C.; Sun, Z.; Zhong, Y.-J.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Li, H.-Y.; Lu, Y.; Zheng, J.-W.; Yang, W.-D.; Liu, J.-S. Biochemical and genetic engineering of diatoms for polyunsaturated fatty acid biosynthesis. Mar. Drugs 2014, 12, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Christensen, J.H.; Aardestrup, I.; Madsen, T.; Riahi, S.; Hansen, V.E.; Skou, H.A. Marine n-3 fatty acids: Basic features and background. Lipids 2001, 36, S65–S68. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.A.; Custodio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-Y.; Chen, F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 2003, 21, 273–294. [Google Scholar] [CrossRef]
- Bourdon, J.A.; Bazinet, T.M.; Arnason, T.T.; Kimpe, L.E.; Blais, J.M.; White, P.A. Polychlorinated biphenyls (PCBs) contamination and aryl hydrocarbon receptor (AHR) agonist activity of omega-3 polyunsaturated fatty acid supplements: Implications for daily intake of dioxins and PCBs. Food Chem. Toxicol. 2010, 48, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Marxen, K.; Schulz, R.; Vanselow, K.H. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar. Drugs 2010, 8, 2526–2545. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-B.; Wang, Z.-Y.; Yu, C.-J.; Yin, Y.-H.; Zhou, G.-K. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, D. Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Bot. J. Linn. Soc. 1981, 82, 93–119. [Google Scholar] [CrossRef]
- Murakami, R.; Hashimoto, H. Unusual nuclear division in Nannochloropsis oculata (Eustigmatophyceae, Heterokonta) which may ensure faithful transmission of secondary plastids. Protist 2009, 160, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef] [PubMed]
- Vieler, A.; Wu, G.; Tsai, C.-H.; Bullard, B.; Cornish, A.J.; Harvey, C.; Reca, I.-B.; Thornburg, C.; Achawanantakun, R.; Buehl, C.J.; et al. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.Y.; Sivaloganathan, B.; Obbard, J.P. Screening of marine microalgae for biodiesel feedstock. Biomass Bioenerg. 2011, 35, 2534–2544. [Google Scholar] [CrossRef]
- Bougaran, G.; Rouxel, C.; Dubois, N.; Kaas, R.; Grouas, S.; Lukomska, E.; Le Coz, J.-R.; Cadoret, J.-P. Enhancement of neutral lipid productivity in the microalga Isochrysis affinis galbana (T-iso) by a mutation-selection procedure. Biotechnol. Bioeng. 2012, 109, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, A.; Narayan, M.S.; Murthy, K.N.C.; Ravishankar, G.A. Comparative study of lipid composition of two halotolerant alga, Dunaliella bardawil and Dunaliella salina. Int. J. Food Sci. Nutr. 2007, 58, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-T.; Tian, F.-L.; Wang, H.-Y.; Hu, Y.-H.; Sheng, W.-L. An application of Dunaliella salina algae: Biodiesel. Adv. Mater. Res. 2014, 953–954, 281–283. [Google Scholar] [CrossRef]
- Cecilia Damiani, M.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Bona, F.; Capuzzo, A.; Franchino, M.; Maffei, M.E. Semicontinuous nitrogen limitation as convenient operation strategy to maximize fatty acid production in Neochloris oleoabundans. Algal Res. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Wu, X.-D.; Ruan, R.-S.; Du, Z.-Y.; Liu, Y.-H. Current status and prospects of biodiesel production from microalgae. Energies 2012, 5, 2667–2682. [Google Scholar] [CrossRef]
- Narayan, M.S.; Manoj, G.P.; Vatchravelu, K.; Bhagyalakshmi, N.; Mahadevaswamy, M. Utilization of glycerol as carbon source on the growth, pigment and lipid production in Spirulina platensis. Int. J. Food Sci. Nutr. 2005, 56, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Lin, Q.; Li, G.; Tan, Y.-H.; Huang, L.-M.; Lin, J.-D. Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng. Life Sci. 2012, 12, 631–637. [Google Scholar] [CrossRef]
- Xu, F.; Cong, W.; Cai, Z.-L.; Ouyang, F. Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 2004, 16, 499–503. [Google Scholar]
- Zittelli, G.C.; Lavista, F.; Bastianini, A.; Rodolfi, L.; Vincenzini, M.; Tredici, M.R. Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J. Biotechnol. 1999, 70, 299–312. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y.; Berner, T. Regulation of fatty acid composition by irradiance level in the Eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar] [CrossRef]
- Kim, C.W.; Sung, M.-G.; Nam, K.; Moon, M.; Kwon, J.-H.; Yang, J.-W. Effect of monochromatic illumination on lipid accumulation of Nannochloropsis gaditana under continuous cultivation. Bioresour. Technol. 2014, 159, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Huang, C.-C.; Lee, W.-L.; Chang, J.-S. Engineering strategies for enhancing the production of eicosapentaenoic acid (EPA) from an isolated microalga Nannochloropsis oceanica CY2. Bioresour. Technol. 2013, 147, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Schenk, P.M. Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnol. Bioeng. 2015, 112, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Rodriguez, J.; Gonzalez-Cespedes, A.M.; Ceron-Garcia, M.C.; Fernandez-Sevilla, J.M.; Acien-Fernandez, F.G.; Molina-Grima, E. A quantitative study of eicosapentaenoic acid (EPA) production by Nannochloropsis gaditana for aquaculture as a function of dilution rate, temperature and average irradiance. Appl. Microbiol. Biotechnol. 2014, 98, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Ho, S.-H.; Chang, J.-S. Enhancing the production of eicosapentaenoic acid (EPA) from Nannochloropsis oceanica CY2 using innovative photobioreactors with optimal light source arrangements. Bioresour. Technol. 2015, 191, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Jiang, J.-P.; Wang, H.-T.; Cao, X.-P.; Xue, S.; Yang, Q.; Wang, W.-L. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Fujita, Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol. Res. 2006, 54, 208–219. [Google Scholar] [CrossRef]
- Xu, F.; Hu, H.-H.; Cong, W.; Cai, Z.-L.; Ouyang, F. Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp. in mixotrophic conditions. Biotechnol. Lett. 2004, 26, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Park, S.Y.; Racharaks, R.; Li, Y.B. Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl. Energy 2013, 108, 486–492. [Google Scholar] [CrossRef]
- Dong, B.-F.; Ho, N.; Ogden, K.L.; Arnold, R.G. Cultivation of Nannochloropsis salina in municipal wastewater or digester centrate. Ecotoxicol. Environ. Saf. 2014, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sheets, J.P.; Ge, X.-M.; Park, S.Y.; Li, Y.-B. Effect of outdoor conditions on Nannochloropsis salina cultivation in artificial seawater using nutrients from anaerobic digestion effluent. Bioresour. Technol. 2014, 152, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-H.; Sun, F.-Q.; Yang, M.; Lu, L.; Yang, G.-P.; Pan, K.-H. Large-scale biodiesel production using flue gas from coal-fired power plants with Nannochloropsis microalgal biomass in open raceway ponds. Bioresour. Technol. 2014, 174, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-L.; Luo, S.-J.; Fan, X.-L.; Yang, Z.-M.; Guo, R.-B. Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl. Energy 2011, 88, 3336–3341. [Google Scholar] [CrossRef]
- Ronda, S.R.; Kethineni, C.; Parupudi, L.C.P.; Thunuguntla, V.B.S.C.; Vemula, S.; Settaluri, V.S.; Allu, P.R.; Grande, S.K.; Sharma, S.; Kandala, C.V. A growth inhibitory model with SOx influenced effective growth rate for estimation of algal biomass concentration under flue gas atmosphere. Bioresour. Technol. 2014, 152, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Thaher, M.I.; Hakim, M.A.Q.M.A.; Al-Jabri, H.M.S.J. Sustainable production of toxin free marine microalgae biomass as fish feed in large scale open system in the Qatari desert. Bioresour. Technol. 2015, 192, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Miyachi, S.; Kurano, N. Evaluation of a vertical flat-plate photobioreactor for outdoor biomass production and carbon dioxide bio-fixation: Effects of reactor dimensions, irradiation and cell concentration on the biomass productivity and irradiation utilization efficiency. Appl. Microbiol. Biotechnol. 2001, 55, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, F. Biology and industrial applications of Chlorella: Advances and prospects. Adv. Biochem. Eng. Biotechnol. 2014, 1–35. [Google Scholar] [CrossRef]
- Briassoulis, D.; Panagakis, P.; Chionidis, M.; Tzenos, D.; Lalos, A.; Tsinos, C.; Berberidis, K.; Jacobsen, A. An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresour. Technol. 2010, 101, 6768–6777. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, P.E.; Drewry, J.L.; Collins, A.M.; Dempster, T.A.; Choi, C.Y.; James, S.C. Analysis and modeling of Nannochloropsis growth in lab, greenhouse, and raceway experiments. J. Appl. Phycol. 2014, 26, 2303–2314. [Google Scholar] [CrossRef]
- Kojima, E.; Zhang, K. Growth and hydrocarbon production of microalga Botryococcus braunii in bubble column photobioreactors. J. Biosci. Bioeng. 1999, 87, 811–815. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Vazhappilly, R.; Chen, F. Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J. Am. Oil Chem. Soc. 1998, 75, 393–397. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, F.; Altimari, P.; Trabucco, F.; Toro, L. Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: Interaction between glucose and nitrate. J. Chem. Technol. Biotechnol. 2014, 89, 652–661. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Sharma, P.K. High-light-induced changes on photosynthesis, pigments, sugars, lipids and antioxidant enzymes in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Photochem. Photobiol. 2006, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Lukyanov, A.; Solovchenko, O.; Didi-Cohen, S.; Boussiba, S.; Khozin-Goldberg, I. Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur. J. Lipid Sci. Technol. 2014, 116, 635–644. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-P.; Liu, J.; Guo, B.-B.; Ma, X.-N.; Sun, P.-P.; Liu, B.; Chen, F. Light attenuates lipid accumulation while enhancing cell proliferation and starch synthesis in the glucose-fed oleaginous microalga Chlorella zofingiensis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Yang, Z.-K.; Niu, Y.-F.; Ma, Y.-H.; Xue, J.; Zhang, M.-H.; Yang, W.-D.; Liu, J.-S.; Lu, S.-H.; Guan, Y.; Li, H.-Y. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.-F.; Liu, T.-Z. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Bartley, M.L.; Boeing, W.J.; Corcoran, A.A.; Holguin, F.O.; Schaub, T. Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenerg. 2013, 54, 83–88. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Optimization of Nannochloropsis oculata growth using the response surface method. J. Chem. Technol. Biotechnol. 2006, 81, 1049–1056. [Google Scholar] [CrossRef]
- Li, J.; Han, D.-X.; Wang, D.-M.; Ning, K.; Jia, J.; Wei, L.; Jing, X.-Y.; Huang, S.; Chen, J.; Li, Y.-T.; Hu, Q.; Xu, J. Choreography of transcriptomes and lipidomes of Nannochloropsis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 2014, 26, 1645–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-M.; Ning, K.; Li, J.; Hu, J.-Q.; Han, D.-X.; Wang, H.; Zeng, X.-W.; Jing, X.-Y.; Zhou, Q.; Su, X.-Q.; et al. Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Muhlroth, A.; Li, K.-S.; Rokke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar. Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Chai, T.J. Reduction in omega-3-fatty-acids by UV-B irradiation in microalgae. J. Appl. Phycol. 1994, 6, 415–421. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Physiological role of neutral lipid accumulation in Eukaryotic microalgae under stresses. Russ. J. Plant Physiol. 2012, 59, 167–176. [Google Scholar] [CrossRef]
- Courchesne, N.M.D.; Parisien, A.; Wang, B.; Lan, C.Q. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 2009, 141, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Adams, I.P.; Ratledge, C. Malic enzyme: The controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiol. Soc. J. 2007, 153, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.-F.; Huang, T.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J. Biotechnol. 2012, 164, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: A reappraisal and unsolved problems. Biotechnol. Lett. 2014, 36, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Huerlimann, R.; Steinig, E.J.; Loxton, H.; Zenger, K.R.; Jerry, D.R.; Heimann, K. The effect of nitrogen limitation on acetyl-CoA carboxylase expression and fatty acid content in Chromera velia and Isochrysis aff. Galbana (Tiso). Gene 2014, 543, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Uppalapati, S.R.; Alamsjah, M.A.; Fujita, Y. Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentaenoic acid following n-methyl-n-nitrosourea-induced random mutagenesis. J. Appl. Phycol. 2004, 16, 135–144. [Google Scholar] [CrossRef]
- Joyard, J.; Ferro, M.; Masselon, C.; Seigneurin-Berny, D.; Salvi, D.; Garin, J.; Rolland, N. Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog. Lipid Res. 2010, 49, 128–158. [Google Scholar] [CrossRef] [PubMed]
- Yongmanitchai, W.; Ward, O.P. Positional distribution of fatty-acids, and molecular-species of polar lipids, in the diatom Phaeodactylum tricornutum. J. Gen. Microbiol. 1993, 139, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-L.; Yu, J.-Z.; Zhu, B.-H.; Pan, K.-H.; Pan, J.; Yang, G.-P. Cloning and characterization of a delta-6 desaturase encoding gene from Nannochloropsis oculata. Chin. J. Oceanol. Limnol. 2011, 29, 290–296. [Google Scholar] [CrossRef]
- Pan, K.-H.; Ma, X.-L.; Yu, J.-Z.; Zhu, B.-H.; Yang, G.-P. Cloning and phylogenetic analysis of a fatty acid elongase gene from Nannochloropsis oculata CS179. J. Ocean Univers. China 2009, 8, 392–398. [Google Scholar] [CrossRef]
- Kaye, Y.; Grundman, O.; Leu, S.; Zarka, A.; Zorin, B.; Didi-Cohen, S.; Khozin-Goldberg, I.; Boussiba, S. Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: Overexpression of endogenous delta 12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal Res. 2015, 11, 387–398. [Google Scholar] [CrossRef]
- Coleman, R.A.; Lee, D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004, 43, 134–176. [Google Scholar] [CrossRef]
- Oelkers, P.; Cromley, D.; Padamsee, M.; Billheimer, J.T.; Sturley, S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002, 277, 8877–8881. [Google Scholar] [CrossRef] [PubMed]
- Sandager, L.; Gustavsson, M.H.; Stahl, U.; Dahlqvist, A.; Wiberg, E.; Banas, A.; Lenman, M.; Ronne, H.; Stymne, S. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 2002, 277, 6478–6482. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, D.-X.; Yoon, K.; Hu, Q.; Li, Y.-T. Characterization of type 2 diacylglycerol acyltransferase in Chlamydomonas reinhardtii reveals their distinct substrate specificities and functions in triacylglycerol biosynthesis. Plant J. 2016. [Google Scholar] [CrossRef]
- Dahlqvist, A.; Stahl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, H. Phospholipid: Diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Specht, M.; Hippler, M. The chloroplast proteome: A survey from the Chlamydomonas reinhardtii perspective with a focus on distinctive features. Curr. Genet. 2011, 57, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Han, D.-X.; Li, Y.-T.; Sommerfeld, M.; Hu, Q. Phospholipid: Diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Browse, J. The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 2011, 68, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Vieler, A.; Brubaker, S.B.; Vick, B.; Benning, C. A lipid droplet protein of Nannochloropsis with functions partially analogous to plant oleosins. Plant Physiol. 2012, 158, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. In Methods in Enzymology, Photosynthesis: Molecular Biology of Energy Capture; Mclntosh, L., Ed.; Academic Press, Inc.: Cambridge, MA, USA, 1998; Volume 297, pp. 27–38. [Google Scholar]
- Chen, H.L.; Li, S.S.; Huang, R.; Tsai, H.-J. Conditional production of a functional fish growth hormone in the transgenic line of Nannochloropsis oculata (Eustigmatophyceae). J. Phycol. 2008, 44, 768–776. [Google Scholar] [CrossRef]
- Kang, N.K.; Lee, B.; Shin, S.-E.; Jeon, S.; Park, M.S.; Yang, J.-W. Use of conditioned medium for efficient transformation and cost-effective cultivation of Nannochloropsis salina. Bioresour. Technol. 2015, 181, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-J.; Gao, D.-W.; Hu, H.-H. High-efficiency nuclear transformation of the oleaginous marine Nannochloropsis species using PCR product. Biosci. Biotech. Biochnol. 2014, 78, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Tsai, H.-J. Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish Immunol. 2009, 26, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-N.; Zhang, X.-W.; Ye, N.-H.; Fan, X.; Mou, S.-L.; Xu, D.; Liang, C.-W.; Wang, Y.-T.; Wang, W.-Q. Evaluation of putative internal reference genes for gene expression normalization in Nannochloropsis sp. by quantitative real-time RT-PCR. Biochem. Biophys. Res. Community 2012, 424, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-W.; Cao, S.-N.; Zhang, X.-W.; Zhu, B.-H.; Su, Z.-L.; Xu, D.; Guang, L.-Y.; Ye, N.-H. De novo sequencing and global transcriptome analysis of Nannochloropsis sp. (Eustigmatophyceae) following nitrogen starvation. Bioenerg. Res. 2013, 6, 494–505. [Google Scholar]




| Species | Total Lipid Content (% of DW) | Neutral Lipid Content (% of Total Lipid) | Ref. |
|---|---|---|---|
| Nannochloropsis | 37–60 | 23–58 | [16] |
| Isochrysis | 25–33 | 80 | [1,22] |
| Dunaliella salina | 23 | 30 | [23,24] |
| Haematococcus pluvialis | 16–35 | 50–59 | [25] |
| Neochloris oleoabundans | 2–47 | 23–73 | [26] |
| Phaeodactylum tricornutum | 20–30 | - | [27] |
| Crypthecodinium cohnii | 20 | - | [1] |
| Spirulina platensis | 7.6–8.2 | - | [28] |
| Tetraselmis maculata | 8 | - | [27] |
| Scenedesmus obliquus | 12–14 | - | [27] |
| Nannochloropsis Species | C14:0 | C16:0 | C16:1 | C16:2 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | C20:4 | C20:5 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. oculata CS179 | 26.7 | 26.6 | 0.6 | 5.9 | 5.3 | 0.1 | 7.1 | 20.2 | [29] | |||
| N. oceanica CCMP531 | 4.5 | 45.9 | 22.7 | 0.6 | 22.2 | 0.7 | 0.5 | 2.5 | 2.9 | [16] | ||
| N. oculata CCMP529 | 2.1 | 29.1 | 28.3 | 1.9 | 22.8 | 2.6 | 1.6 | 6.2 | 5.4 | [16] | ||
| N. limnetica CCMP505 | 16.6 | 2.9 | 3.1 | 4.6 | 31.5 | 23.8 | 17.5 | [16] | ||||
| N. granulata CCMP525 | 2.4 | 26.2 | 24.0 | 3.3 | 28.5 | 4.7 | 1.6 | 4.5 | 4.8 | [16] | ||
| N. gaditana CCMP527 | 2.7 | 39.2 | 24.1 | 3.1 | 14.2 | 4.7 | 1.6 | 4.5 | 4.7 | [16] | ||
| N. salina CCMP537 | 3.3 | 32.2 | 25.4 | 3.0 | 2.5 | 15.5 | 3.0 | 0.6 | 3.6 | 10.9 | [16] | |
| N. salina CCMP1176 | 2.1 | 32.0 | 30.0 | 3.2 | 9.4 | 2.6 | 0.9 | 7.2 | 12.7 | [16] | ||
| Nannochloropsis sp. | 4.3 | 24.6 | 30.2 | 1.1 | 11.0 | 1.9 | 1.5 | 21.8 | [30] | |||
| Nannochloropsis sp. | 4.0 | 21.8 | 25.8 | 4.2 | 2.5 | 6.5 | 33.7 | [31] |
| Species | EPA Content (% DW) | Ref. |
|---|---|---|
| N. gaditana | 4.3 | [37] |
| N. oceanica CY2 | 4.4–5.1 | [38] |
| N. oceanica CY2 | 5.5 | [35] |
| N. salina | 1.1–3.5 | [15] |
| N. oceanica IMET1 | 2.7–5.2 | [39] |
| N. oculata | 2–3 | [40] |
| Nannochloropsis sp. | 12 | [36] |
| Nannochloropsis sp. | 5–6 | [41] |
| Nannochloropsis sp. | 3–6 | [30] |
| Nannochloropsis sp. | 4 | [31] |
| Nannochloropsis sp. | 2–4 | [42] |
| Nannochloropsis Species | Waste Water Sources | Flue Gas Sources | Specific Growth Rate (/day) | Lipid Content (% DW) | Ref. |
|---|---|---|---|---|---|
| N. salina | Anaerobic digestion effluent | 0.3–0.6 | 21–36 | [43] | |
| N. salina | Wastewater Reclamation Facility/ Stream from dewatering anaerobically digested sludge | [44] | |||
| N. salina | Anaerobic digestion effluent | 0.04–0.15 | 26–32 | [45] | |
| N. oceanica | Coal-fired power plants | 0.05–0.07 | 21–28 | [46] | |
| Nannochloropsis sp. | Municipal wastewater | Filtered compressed gas (15% CO2) | 0.52 | 33 | [47] |
| N. limnetica | Rice husk emission | [48] |
| Nannochloropsis Species | Reporter or Marker Gene | Promoter | Selection | Transformation Method | Ref. |
|---|---|---|---|---|---|
| N. gaditana | Bleomycin resistance | TUB, UEP, HSP | Bleomycin | Electroporation | [19] |
| N. salina | GUS | TUB | Zeocin | Electroporation | [99] |
| N. oculata | Fish growth hormone | HSP, RUBISCO SSU 2 | Ampicillin | Electroporation | [97] |
| N. oculata | Bovine lactoferricin | HSP, RUBISCO | Electroporation | [100] | |
| N. oceanica | Δ12 desaturase | LDSP | Uracil auxotrophy | Electroporation | [86] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. https://doi.org/10.3390/md14040061
Ma X-N, Chen T-P, Yang B, Liu J, Chen F. Lipid Production from Nannochloropsis. Marine Drugs. 2016; 14(4):61. https://doi.org/10.3390/md14040061
Chicago/Turabian StyleMa, Xiao-Nian, Tian-Peng Chen, Bo Yang, Jin Liu, and Feng Chen. 2016. "Lipid Production from Nannochloropsis" Marine Drugs 14, no. 4: 61. https://doi.org/10.3390/md14040061
APA StyleMa, X. -N., Chen, T. -P., Yang, B., Liu, J., & Chen, F. (2016). Lipid Production from Nannochloropsis. Marine Drugs, 14(4), 61. https://doi.org/10.3390/md14040061