Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer
Abstract
:1. Introduction
2. Results
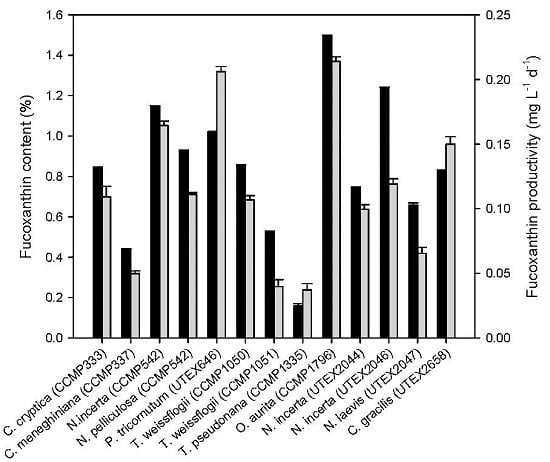
2.1. Fucoxanthin Production of Diatoms under Photoautotrophic Conditions
2.2. Growth and Fucoxanthin Production Potential of Diatoms under Heterotrophic Conditions
2.3. Effect of Nitrate and/or Light Supply on Growth and Fucoxanthin Production of C. cryptica
2.4. Effect of Light Intensity on Growth and Fucoxanthin Production
2.5. Effect of Light Intensity on Diadinoxanthin and Diatoxanthin
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Determination of Biomass Concentration and Fucoxanthin Production
4.3. Pigments Extraction
4.4. Analysis of Pigments by HPLC and LC-MS
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo Sci. 2007, 56, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res. 2012, 32, 807–813. [Google Scholar] [PubMed]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Ahn, G.; Heo, S.J.; Kang, S.M.; Kang, M.C.; Yang, H.M.; Kim, D.; Roh, S.W.; Kim, S.K.; Jeon, B.T. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. BBA Gen. Subj. 2008, 1780, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishida, M.; Nishi, K.; Oda, H.; Toyohara, H.; Sugahara, T. Studies on anti-allergic activity of Sargassum horneri extract. J. Funct. Foods 2014, 10, 154–160. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, M.; López, D.P.; Sáiz, M.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354. [Google Scholar] [CrossRef]
- Fourest, E.; Volesky, B. Alginate properties and heavy metal biosorption by marine algae. Appl. Biochem. Biotechnol. 1997, 67, 215–226. [Google Scholar] [CrossRef]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Metabolic insertion of nanostructured TiO2 into the patterned biosilica of the diatom Pinnularia sp. by a two-stage bioreactor cultivation process. ACS Nano 2008, 2, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. A perfusion-cell bleeding culture strategy for enhancing the productivity of eicosapentaenoic acid by Nitzschia laevis. Appl. Microbiol. Biotechnol. 2001, 57, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Mooij, P.; van Loosdrecht, M.; Kleerebezem, R. Storage Compound Production by Phototrophic Diatoms; European Patent Office: Delft, The Nertherland, 2015. [Google Scholar]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Lewin, J.C. Heterotrophy in Diatoms. J. Gen. Microbiol. 1953, 9, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom Nitzschia laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Stransky, H.; Hager, A. The carotenoid pattern and the occurrence of the light-induced xanthophyll cycle in various classes of algae. VI. Chemosystematic study. Arch. Mikrobiol. 1969, 73, 315–323. [Google Scholar] [CrossRef]
- Kolber, Z.; Zehr, J.; Falkowski, P. Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol. 1988, 88, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. P. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Reddy, C.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. J. Appl. Phycol. 2008, 20, 609–617. [Google Scholar] [CrossRef]
- Coesel, S.; Oborník, M.; Varela, J.; Falciatore, A.; Bowler, C. Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, e2896. [Google Scholar] [CrossRef] [PubMed]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of some environmental factors. J. Biosci. Bioeng. 2010, 109, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M.; Roche, J.L.; Kolber, Z.; Falkowski, P.G. Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth. Res. 1994, 41, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Wilhelm, C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. USA 1999, 96, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M.; Yamamoto, H.Y. Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae). J. Phycol. 1994, 30, 606–612. [Google Scholar] [CrossRef]
- Piovan, A.; Seraglia, R.; Bresin, B.; Caniato, R.; Filippini, R. Fucoxanthin from Undaria pinnatifida: Photostability and coextractive effects. Molecules 2013, 18, 6298–6310. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.; Yau, S.K. Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]
- Lewin, J.; Hellebust, J.A. Heterotrophic nutrition of the marine pennate diatom, Cylindrotheca fusiformis. Can. J. Microbiol. 1970, 16, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.; Weesepoel, Y.; Lamers, P.P.; Vincken, J.-P.; Martens, D.E.; Wijffels, R.H. Growth and pigment accumulation in nutrient-depleted Isochrysis aff. galbana T-ISO. J. Appl. Phycol. 2013, 25, 1421–1430. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Lewin, J.C.; Lewin, R.A. Auxotrophy and heterotrophy in marine littoral diatoms. Can. J. Microbiol. 1960, 6, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, F. Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: Effects of silicate and glucose. J. Ind. Microbiol. Biotechnol. 2000, 25, 218–224. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. Glucose sensing and the mitochondrial alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (Chlorophyceae). Planta 2008, 228, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Syrett, P.; Wong, H.-A. The fermentation of glucose by Chlorella vulgaris. Biochem. J. 1963, 89, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; del Valle, E.M.M.; Galán, M.A. Evaluating the industrial potential of biodiesel from a microalgae heterotrophic culture: Scale-up and economics. Biochem. Eng. J. 2012, 63, 104–115. [Google Scholar] [CrossRef]
- Vazhappilly, R.; Chen, F. Heterotrophic production potential of omega-3 polyunsaturated fatty acids by microalgae and algae-like microorganisms. Bot. Mar. 1998, 41, 553–558. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; King, K.D.; Chen, F. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of nitrogen source and concentration. J. Appl. Phycol. 2012, 24, 301–307. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Fidalgo, J.P.; Cid, A.; Torres, E.; Sukenik, A.; Herrero, C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 1998, 166, 105–116. [Google Scholar] [CrossRef]
- DeBoer, J.A.; Guigli, H.J.; Israel, T.L.; D’Elia, C.F. Nutritional studies of two red algae. I. Growth rate as a function of nitrogen source and concentration. J. Phycol. 1978, 14, 261–266. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 2015, 66, 6281–6296. [Google Scholar] [CrossRef] [PubMed]
- Willemoës, M.; Monas, E. Relationship between growth irradiance and the xanthophyll cycle pool in the diatom Nitzschia palea. Physiol. Plant. 1991, 83, 449–456. [Google Scholar] [CrossRef]
- Van de Poll, W.H.; van Leeuwe, M.A.; Roggeveld, J.; Buma, A.G. Nutrient limitation and high irradiance acclimation reduce PAR and UV-induced viability loss in the antarctic diatom Chaetoceros brevis (Bacillariophyceae). J. Phycol. 2005, 41, 840–850. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2015, 1–12. [Google Scholar] [CrossRef]
- Chen, T.; Liu, J.; Guo, B.; Ma, X.; Sun, P.; Liu, B.; Chen, F. Light attenuates lipid accumulation while enhancing cell proliferation and starch synthesis in the glucose-fed oleaginous microalga Chlorella zofingiensis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]







| Peak No. | Compound | tr * (min) | λmax (nm) | Ionization Type | Mass Spectra M+ m/z (Relative Intensity, %) |
|---|---|---|---|---|---|
| 2 | Diadinoxanthin | 21.059 | 444.9 475.3 | [M+H]+ [M+H-H2O]+ | 565.4(10); 583.4(100); 584.4(50); 585.4(10) |
| 3 | Diatoxanthin | 26.257 | 449.8 478.9 | [M+H]+ [M+H-H2O]+ | 549.4(10); 567.4(100); 568.4(50); 569.4(10) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. https://doi.org/10.3390/md14070125
Guo B, Liu B, Yang B, Sun P, Lu X, Liu J, Chen F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Marine Drugs. 2016; 14(7):125. https://doi.org/10.3390/md14070125
Chicago/Turabian StyleGuo, Bingbing, Bin Liu, Bo Yang, Peipei Sun, Xue Lu, Jin Liu, and Feng Chen. 2016. "Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer" Marine Drugs 14, no. 7: 125. https://doi.org/10.3390/md14070125
APA StyleGuo, B., Liu, B., Yang, B., Sun, P., Lu, X., Liu, J., & Chen, F. (2016). Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Marine Drugs, 14(7), 125. https://doi.org/10.3390/md14070125