Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Compounds 1–9
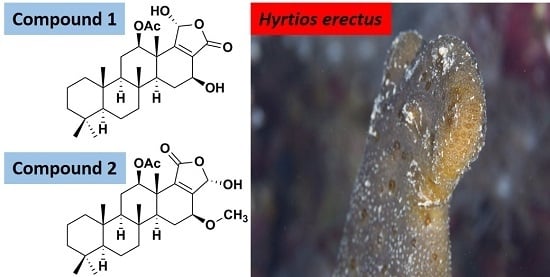
2.2. Structure Elucidation of Compounds 1–9
2.3. Biological Activities of the Isolated Compounds
2.3.1. Antiproliferative Assessment of Compounds 1–9
2.3.2. Programmed Cell Death Induced by Compounds 3, 5 and 9 against HCT-116 Cells
2.3.3. Influence of Compounds 3, 5 and 9 on Cell Cycle Distribution of HCT-116 Cells
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Purification of Compounds 1–9
3.4. Biological Activity of Compounds 1–9
3.4.1. Cell Culture
3.4.2. Trypan-Blue Exclusion Assay
3.4.3. Antiproliferative Assessment
3.4.4. Apoptosis Assessment Using Annexin V-FITC Staining Coupled with Flowcytometry
3.4.5. Analysis of Cell Cycle Distribution
3.4.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can Some Marine-Derived Fungal Metabolites Become Actual Anticancer Agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Yamaki, R.K.; Kelly, M.; Scheuer, P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002, 65, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Tan, R.; Cichacz, Z.A. Antineoplastic agents. 542. Isolation and structure of sesterstatin 6 from the Indian Ocean sponge Hyrtios erecta. J. Nat. Prod. 2005, 68, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Cichacz, Z.A.; Tan, R.; Hoard, M.S.; Melody, N.; Pettit, R.K. Antineoplastic agents. 386. Isolation of sesterstatins 1–3 from the marine sponge Hyrtios erecta. J. Nat. Prod. 1998, 61, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Matsunaga, S.; Fusetani, N. Three new cytotoxic sesterterpenes from the marine sponge Hyrtios cf. erectus. J. Nat. Prod. 1996, 59, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Nasu, S.S.; Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M.; Goins, K. Puupehenone-related metabolites from two Hawaiian sponges, Hyrtios spp. J. Org. Chem. 1995, 60, 7290–7292. [Google Scholar] [CrossRef]
- Pettit, G.R.; Cichaz, Z.A.; Tan, R.; Herald, D.L.; Melody, N.; Hoard, M.S.; Doubek, D.L.; Hooper, J.N.A. Antineoplastic agents. 385. The isolation and structure of a scalarane-type sesterterpene from the Indian Ocean porifera Hyrtios erecta. Collect. Czech. Chem. Commun. 1998, 63, 1671–1677. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Melody, N.; Cichacz, Z.A.; Herald, D.L.; Hoard, M.S.; Pettit, R.K.; Chapuis, J.C. Antineoplastic agents. 397: Isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (the Republic of Maldives). Bioorg. Med. Chem. Lett. 1998, 8, 2093–2098. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Lefranc, F.; Cimmino, A.; Dasari, R.; Evidente, M.; Mathieu, V.; Kiss, R. Sesterterpenoids with Anticancer Activity. Curr. Med. Chem. 2015, 22, 3502–3522. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A.; Emara, S. Antimycobacterial scalarane-based sesterterpenes from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2005, 68, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Hochlowski, J.E.; Faulkner, D.J.; Bass, L.S.; Clardy, J. Metabolites of the dorid nudibranch Chromodoris sedna. J. Org. Chem. 1983, 48, 1738–1740. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cambie, R.C.; Kernan, M.R. Scalarane sesterterpenes from Collospongia auris, a new thorectid sponge. Biochem. Syst. Ecol. 1990, 18, 349–357. [Google Scholar] [CrossRef]
- Kikuchi, H.; Tsukitani, Y.; Shimizu, I.; Kobayashi, M.; Kitagawa, I. Marine Natural Products. XI. An Antiinflammatory Scalarane-type Bishomosesterterpene, Foliaspongin, from the Okinawan Marine Sponge Phyllospongia foliascens (PALLAS). Chem. Pharm. Bull. 1983, 31, 552–556. [Google Scholar] [CrossRef]
- Crews, P.; Bescansa, P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986, 49, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Thompson, J.E.; Faulkner, D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980, 45, 4976–4979. [Google Scholar] [CrossRef]
- Terem, B.; Scheuer, P.J. Scalaradial derivatives from the nudibranch chromodoris youngbleuthi and the sponge spongia oceania. Tetrahedron 1986, 42, 4409–4412. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Kaisin, M.; Moussiaux, B. Ichthyotoxic sesterterpenoids from the neo guinean sponge carteriospongia foliascens. Tetrahedron 1985, 41, 4603–4614. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.; Wells, R. Five new C26 tetracyclic terpenes from a sponge (Lendenfeldia sp.). Aust. J. Chem. 1982, 35, 51–59. [Google Scholar] [CrossRef]
- Nakagawa, M.; Hamamoto, Y.; Ishihama, M.; Hamasaki, S.; Endo, M. Pharmacologically active homosesterterpenes from palauan sponges. Tetrahedron Lett. 1987, 28, 431–434. [Google Scholar] [CrossRef]
- De Cook, S.; Bergquist, P. Family Thorectidae Bergquist, 1978. In Systema Porifera; Hooper, J.A., van Soest, R.M., Willenz, P., Eds.; Springer US: New York, NY, USA, 2002; pp. 1028–1050. [Google Scholar]
- Qiu, Y.; Deng, Z.; Pei, Y.; Fu, H.; Li, J.; Proksch, P.; Lin, W. Sesterterpenoids from the marine sponge Hyrtios erectus. J. Nat. Prod. 2004, 67, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, H.; Nishijima, S.; Mitome, H.; Yamada, Y. Three new scalarane sesterterpenoids from the Okinawan sponge Hyrtios erectus. J. Nat. Prod. 2000, 63, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Singab, A.N.B.; van Soest, R.W.M.; Fusetani, N. Hyrtiosenolides A and B, Two New Sesquiterpene γ-Methoxybutenolides and a New Sterol from a Red Sea Sponge Hyrtios Species. J. Nat. Prod. 2004, 67, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Piña, I.C.; Sanders, M.L.; Crews, P. Puupehenone Congeners from an Indo-Pacific Hyrtios Sponge. J. Nat. Prod. 2003, 66, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J.C.; Gomez, R.; de Kluijver, M.; Van Soest, R.W.M. New Sesquiterpene/Quinones from Two Sponges of the Genus Hyrtios. J. Nat. Prod. 2000, 63, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Aoki, S.; Sakai, H.; Kawazoe, K.; Kihara, N.; Sasaki, T.; Kitagawa, I. Altohyrtin A, a potent anti-tumor macrolide from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1993, 34, 2795–2798. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Sakai, H.; Kihara, N.; Sasaki, T.; Kitagawa, I. Altohyrtins B and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolide congeners of Altohyrtin A, from the okinawan marine sponge hyrtios altum. Chem. Pharm. Bull. 1993, 41, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A. Hyrtioerectines AC, cytotoxic alkaloids from the Red Sea sponge Hyrtios erectus. J. Nat. Prod. 2005, 68, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Sauleau, P.; Martin, M.T.; Dau, M.E.T.H.; Youssef, D.T.A.; Bourguet-Kondracki, M.L. Hyrtiazepine, an Azepino-indole-Type Alkaloid from the Red Sea Marine Sponge Hyrtios erectus. J. Nat. Prod. 2006, 69, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J.C.; van Soest, R.W.M. 5-Hydroxytryptamine-derived alkaloids from two marine sponges of the genus Hyrtios. J. Nat. Prod. 2002, 65, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Ye, Y.; Higuchi, K.; Takashima, A.; Tanaka, Y.; Kitagawa, I.; Kobayashi, M. Novel neuronal nitric oxide synthase (nNOS) selective inhibitors, aplysinopsin-type indole alkaloids, from marine sponge Hyrtios erecta. Chem. Pharm. Bull. 2001, 49, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Murayama, T.; Ishibashi, M.; Kosuge, S.; Takamatsu, M.; Ohizumi, Y.; Kobayashi, H.; Ohta, T.; Nozoe, S.; Sasaki, T. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron 1990, 46, 7699–7702. [Google Scholar] [CrossRef]
- Elhady, S.; El-Halawany, A.; Alahdal, A.; Hassanean, H.; Ahmed, S. A New Bioactive Metabolite Isolated from the Red Sea Marine Sponge Hyrtios erectus. Molecules 2016, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Mahidol, C.; Prawat, H.; Sangpetsiripan, S.; Ruchirawat, S. Bioactive Scalaranes from the Thai Sponge Hyrtios gumminae. J. Nat. Prod. 2009, 72, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Murphy, P.; Quinn, R.; Wells, R. Heteronemin, a new scalarin type sesterterpene from the sponge Heteronema erecta. Tetrahedron Lett. 1976, 17, 2631–2634. [Google Scholar] [CrossRef]
- Cimino, G.; de Stefano, S.; Minale, L.; Trivellone, E. 12-epi-Scalarin and 12-epi-deoxoscalarin, sesterterpenes from the sponge Spongia nitens. J. Chem. Soc. Perkin Trans. 1977, 1, 1587–1593. [Google Scholar] [CrossRef]
- Jeon, J.E.; Bae, J.; Lee, K.J.; Oh, K.B.; Shin, J. Scalarane Sesterterpenes from the Sponge Hyatella sp. J. Nat. Prod. 2011, 74, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Mendola, D. Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: Process developments and economics. Biomol. Eng. 2003, 20, 441–458. [Google Scholar] [CrossRef]
- Norcross, R.D.; Paterson, I. Total synthesis of bioactive marine macrolides. Chem. Rev. 1995, 95, 2041–2114. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P. Synthetic studies of biologically active marine cyclopeptides. Chem. Rev. 1995, 95, 2115–2134. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al-Abd, A.M.; Lightfoot, D.A.; El-Shemy, H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzym. Inhib. Med. Chem. 2012, 27, 673–679. [Google Scholar] [CrossRef] [PubMed]







| Position | δC | δH (m, J in Hz) | HMBC (H→C) a |
|---|---|---|---|
| 1 | 39.7, CH2 | 1.66, 0.79 (m) | C-10 |
| 2 | 18.5, CH2 | 1.60, 1.44 (m) | C-4, C-10 |
| 3 | 41.9 CH2 | 1.37, 1.11 (m) | C-4 |
| 4 | 33.3 qC | - | - |
| 5 | 56.5 CH | 0.78 (m) | C-4 |
| 6 | 18.0 CH2 | 1.59, 1.39 (m) | |
| 7 | 41.4 CH2 | 1.78, 0.92 (m) | C-8 |
| 8 | 37.3 qC | - | - |
| 9 | 58.3 CH | 0.89 (m) | C-10, C-12 |
| 10 | 37.4 qC | - | - |
| 11 | 25.8 CH2 | 1.82, 1.55 (m) | C-10, C-12 |
| 12 | 73.8 CH | 3.82 (dd, 16.8, 6.6) | C-9, C-11, C-13, C-25, C-26 |
| 13 | 44.6 qC | - | - |
| 14 | 53.3 CH | 1.25 (m) | C-8, C-9, C-13, C-16, C-18 |
| 15 | 24.2 CH2 | 2.20, 1.63 (m) | C-8, C-13, C-14, C-16, C-17 |
| 16 | 65.9 CH | 5.67 (dd, 9.6, 7.2) | C-15, C-17, C-20 |
| 17 | 126.1 qC | - | - |
| 18 | 168.7 qC | - | - |
| 19 | 95.5 CH | 6.17 (s) | C-17, C-18 |
| 20 | 170.8 qC | - | - |
| 21 | 21.2 CH3 | 0.80 (s) | C-4 |
| 22 | 33.2 CH3 | 0.84 (s) | C-4 |
| 23 | 17.5 CH3 | 0.89 (s) | C-7, C-8, C-9, C-14 |
| 24 | 16.1 CH3 | 0.84 (s) | C-1, C-5, C-9, C-10 |
| 25 | 16.7 CH3 | 1.23 (s) | C-12, C-13, C-14, C-18 |
| 26 | 169.8 qC | - | - |
| 27 | 21.0 CH3 | 2.09 (s) | C-26 |
| Position | δC | δH (m, J in Hz) | HMBC (H→C) a |
|---|---|---|---|
| 1 | 39.5, CH2 | 1.61, 0.82 (m) | C-10 |
| 2 | 18.4, CH2 | 1.60, 1.42 (m) | C-4, C-10 |
| 3 | 42.0 CH2 | 1.36, 1.11 (m) | C-4 |
| 4 | 33.2 qC | - | - |
| 5 | 56.5 CH | 0.79 (m) | C-4 |
| 6 | 18.1 CH2 | 1.58, 1.42 (m) | - |
| 7 | 41.6 CH2 | 1.84, 0.93 (m) | C-8 |
| 8 | 37.1 qC | - | - |
| 9 | 57.7 CH | 0.96 (m) | C-10, C-12 |
| 10 | 37.3 qC | - | - |
| 11 | 24.4 CH2 | 1.74, 1.55 (m) | C-10, C-12 |
| 12 | 75.9 CH | 4.88 (dd, 10.8, 3.6) | C-13, C-18, C-25, C-26 |
| OH | - | 4.25 (br s) | - |
| 13 | 41.5 qC | - | - |
| 14 | 54.2 CH | 1.15 (m) | C-25 |
| 15 | 23.2 CH2 | 2.29, 1.48 (m) | - |
| 16 | 74.5 CH | 4.08 (dd, 9.0, 6.6) | C-17, C-18, C-28 |
| 17 | 159.4 qC | - | - |
| 18 | 138.3 qC | - | - |
| 19 | 167.8 qC | - | - |
| 20 | 94.2 CH | 5.98 (s) | C-17, C-18, C-19 |
| 21 | 21.2 CH3 | 0.80 (s) | C-3, C-4, C-5 |
| 22 | 33.3 CH3 | 0.84 (s) | C-3, C-4, C-5, C-21 |
| 23 | 17.4 CH3 | 0.92 (s) | C-7, C-8, C-9, C-14 |
| 24 | 15.9 CH3 | 0.82 (s) | C-1, C-5, C-9, C-10 |
| 25 | 16.6 CH3 | 1.26 (s) | C-12, C-13, C-14, C-18 |
| 26 | 171.9 qC | - | - |
| 27 | 21.8 CH3 | 2.13 (s) | C-27 |
| 28 | 57.5 OCH3 | 3.47 (s) | C-16 |
| Cell Type | Cell Line | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Breast | MCF-7 | 32.6 | 40.3 | 12.7 | 24.0 | 1.1 | >100 | 30.7 | 20.9 | 3.3 |
| Colorectal | HCT-116 | 57.5 | 22.5 | 3.5 | 26.1 | 0.7 | >100 | 14.4 | 15.4 | 3.4 |
| Hepatocellular | HepG2 | 21.8 | 42.5 | 9.6 | 19.2 | 1.1 | >100 | 17.3 | 15.5 | 1.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhady, S.S.; Al-Abd, A.M.; El-Halawany, A.M.; Alahdal, A.M.; Hassanean, H.A.; Ahmed, S.A. Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus. Mar. Drugs 2016, 14, 130. https://doi.org/10.3390/md14070130
Elhady SS, Al-Abd AM, El-Halawany AM, Alahdal AM, Hassanean HA, Ahmed SA. Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus. Marine Drugs. 2016; 14(7):130. https://doi.org/10.3390/md14070130
Chicago/Turabian StyleElhady, Sameh S., Ahmed M. Al-Abd, Ali M. El-Halawany, Abdulrahman M. Alahdal, Hashim A. Hassanean, and Safwat A. Ahmed. 2016. "Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus" Marine Drugs 14, no. 7: 130. https://doi.org/10.3390/md14070130
APA StyleElhady, S. S., Al-Abd, A. M., El-Halawany, A. M., Alahdal, A. M., Hassanean, H. A., & Ahmed, S. A. (2016). Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus. Marine Drugs, 14(7), 130. https://doi.org/10.3390/md14070130