Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology
Abstract
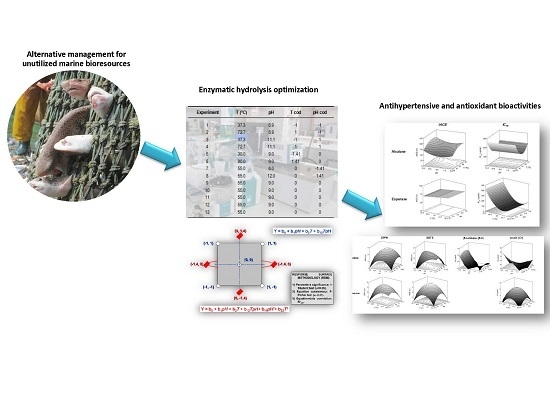
:1. Introduction
2. Results and Discussion
2.1. Composition of S. canicula Muscle
2.2. Hydrolysis Process and Production of FPH
2.3. Determination of the In Vitro Antihypertensive Activities of the Produced FPH
2.4. Determination of Antioxidant Activities of the Produced FPH
3. Materials and Methods
3.1. S. canicula Discards
Proximate Composition of S. canicula Muscle
3.2. Experimental Design
| the model is acceptable when | |
| F1 = Model/Total error | |
| F2 = (Model + Lack of fitting)/Model | |
| F3 = Total error/Experimental error | |
| F4 = Lack of fitting/Experimental error |
3.3. Enzyme Proteolysis of Muscle Discards
3.4. Antihypertensive Activities and Angiotensin I-Converting Enzyme (ACE) Inhibition Assay
3.5. Antioxidant Activity Determinations
3.5.1. 1,1-Diphenyl-2-Picryhydrazyl (DPPH) Radical-Scavenging Capacity
3.5.2. ABTS Bleaching Method
3.5.3. β-Carotene Bleaching Method
3.5.4. Crocin Bleaching Method
3.6. Numerical and Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodríguez-Cabello, C.; Fernández, A.; Olaso, I.; Sánchez, F.; Gancedo, R.; Punzón, A.; Cendrero, O. Overview of continental shelf elasmobranch fisheries in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 375–385. [Google Scholar]
- Blanco, M. Valorización de Descartes y Subproductos de Pintarroja (Scyliorhinus canicula). Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2015. [Google Scholar]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. Hydrolysis as a valorization strategy for unused marine food biomass: Boarfish and small-spotted catshark discards and by-products. J. Food Biochem. 2015, 39, 368–376. [Google Scholar] [CrossRef]
- Roblet, C.; Akhartar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhanceent of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from salmon frame protein hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidante activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Pires, C.; Clement, T.; Batista, I. Functional properties of protein hydrolysates from Cape hake by-products prepared by three different methodologies. J. Sci. Food Agric. 2013, 93, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidante peptides from protein hydrolysates of bluefin leatherjacket (Novadon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Multiobjective optimization of the antioxidant activities of horsemackerel hydrolysates produced with protease mixtures. Process Biochem. 2017, 52, 149–158. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Production and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides from Mediterranean fish discards. J. Funct. Foods 2015, 18, 95–105. [Google Scholar]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar. Drugs 2015, 13, 6537–6549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.; Simpson, B.K.; Pérez-Martín, R.I.; Sotelo, C.G. Isolation and partial characterization of trypsin from pancreas of small-spotted catshark (Scyliorhinus canicula). J. Food Biochem. 2014, 38, 196–206. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ariza, P.R.; Pérez-Martín, R.I. Characterization of collagen from different discarded fish species of the west coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef]
- Silva, J.L.; Chamul, R. Composition of marine and freshwater finfish and shellfish species and their products. In Marine and Freshwater Products Handbook; Martin, R.E., Carter, E.P., Flick, G.J., Davis, L.M., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 31–46. [Google Scholar]
- Bougatef, A.; Nedjar-Arroume, N.; Ravallec-Plé, R.; Leroy, Y.; Guillochon, D.; Barkia, A.; Nasri, M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008, 111, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.M.; Martin, A.M. Optimization of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) protein. Composition of the hydrolysates. Int. J. Food Sci. Nutr. 2009, 48, 191–200. [Google Scholar] [CrossRef]
- Batista, I.; Ramos, C.; Coutinho, J.; Bandarra, N.M.; Nunes, M.L. Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochem. 2010, 45, 18–24. [Google Scholar] [CrossRef]
- Mosquera, M.; Giménez, B.; Da Silva, I.M.; Boelter, J.F.; Montero, P.; Gómez-Guillén, M.C.; Brandelli, A. Nanoencapsulation of an active peptidic fraction from sea bream scales collagen. Food Chem. 2014, 156, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cinq-Mars, C.D.; Li-Chan, E.C.Y. Optimizing angiotensin I-convertingenzyme inhibitory activity of pacific hake (Merluccius productus) fillet hydrolysate using response surface methodology and ultrafiltration. J. Agric. Food Chem. 2007, 55, 9380–9388. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hur, S.; Choi, B.D.; Konno, K.; Park, J.W. Enzymatic hydrolysis of recovered protein from frozen small croaker and functional properties of its hydrolysates. J. Food Sci. 2009, 74, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rial, D.; Vázquez, J.A.; Menduiña, A.; García, A.M.; González, M.P.; Mirón, J.; Murado, M.A. Toxicity of binary mixtures of oil fractions to sea urchin embryos. J. Hazard. Mater. 2013, 263, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R.I. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ovissipour, M.; Rasco, B.; Shiroodi, S.G.; Modanlow, M.; Gholami, S.; Nemati, M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J. Food Sci. Agric. 2013, 93, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Aguilar, R.; Mazorra-Manzano, M.A.; Ramirez-Suarez, J.C. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008, 109, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Nurdiani, R.; Dissanayake, M.; Street, W.E.; Donkor, O.N.; Singh, T.K.; Vasiljevic, T. In vitro study of selected physiological and physicochemical properties of fish protein hydrolysates from 4 Australian fish species. Int. Food Res. J. 2016, 23, 2042–2053. [Google Scholar]
- Estévez, N.; Fuciños, P.; Sobrosa, A.C.; Pastrana, L.; Pérez, N.; Rúa, M.L. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration-response curves. Biotechnol. Prog. 2012, 28, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Ruiz-Quesada, C.; Pérez-Morilla, A.I.; Martínez-Agustín, O.; Guadix, A.; Guadix, E.M. Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: Effect of enzymatic treatment and degree of hydrolysis. J. Sci. Food Agric. 2017, 97, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Salampessy, J.; Reddy, N.; Kailasapathy, K.; Phillips, M. Functional and potential therapeutic ACE-inhibitory peptides derived from bromelain hydrolysis of trevally proteins. J. Funct. Foods 2015, 14, 716–725. [Google Scholar] [CrossRef]
- Nasri, R.; Younes, I.; Jridi, M.; Trigui, M.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Nasri, M.; Karra-Châabouni, M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophio-cephalus) fish protein hydrolysates: Effect on meat lipid oxidation. Food Res. Int. 2013, 54, 552–561. [Google Scholar] [CrossRef]
- Aissaoui, N.; Abidi, F.; Marzouki, M.N. ACE inhibitory and antioxidant activities of red scorpionfish (Scorpaena notata) protein hydrolysates. J. Food Sci. Technol. 2015, 52, 7092–7102. [Google Scholar] [CrossRef]
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 2016, 23, 24901–24911. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Adler-Nissen, J. Enzymatic Hydrolysis of Food Proteins; Elsevier: London, UK, 1986; pp. 132–142. [Google Scholar]
- Amado, I.R.; González, M.P.; Murado, M.A.; Vázquez, J.A. Shrimp wastewater as a source of astaxanthin and bioactive peptides. J. Chem. Technol. Biotechnol. 2016, 91, 793–805. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Vázquez, J.A.; Murado, M.A. Crocin bleaching antioxidant assay revisited. Application to microplate to analyse antioxidant and prooxidant activities. Food Chem. 2015, 167, 299–310. [Google Scholar] [CrossRef] [PubMed]



| Experimental Conditions | Hm (%) | vm (% min−1) | τ (min) | β | R2 | p-Value | |
|---|---|---|---|---|---|---|---|
| Alcalase | T:−1 (37.3 °C)/pH:−1 (6.9) | 11.39 ± 0.32 | 0.068 ± 0.006 | 42.41 ± 2.95 | 0.73 ± 0.05 | 0.982 | <0.001 |
| T:1 (72.7 °C)/pH:−1 (6.9) | 13.52 ± 0.01 | 0.811 ± 0.012 | 4.08 ± 0.09 | 0.71 ± 0.01 | 0.999 | <0.001 | |
| T:−1 (37.3 °C)/pH:1 (11.1) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:1 (72.7 °C)/pH:1 (11.1) | 29.26 ± 1.34 | 0.063 ± 0.005 | 122.2 ± 11.6 | 0.76 ± 0.03 | 0.998 | <0.001 | |
| T:−1.41 (30.0 °C)/pH:0 (9.0) | 12.57 ± 0.29 | 0.053 ± 0.003 | 75.53 ± 3.22 | 0.92 ± 0.04 | 0.991 | <0.001 | |
| T:1.41 (80.0 °C)/pH:0 (9.0) | 12.79 ± 9.30 | 0.002 ± 0.001 | 826.5 (NS) | 0.34 ± 0.06 | 0.966 | <0.001 | |
| T:0 (55.0 °C)/pH:−1.41 (6.0) | 5.61 ± 0.01 | 1.67 ± 0.30 | 0.44 ± 0.11 | 0.38 ± 0.03 | 0.974 | <0.001 | |
| T:0 (55.0 °C)/pH:1.41 (12.0) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:0 (55.0 °C)/pH:0 (9.0) | 21.66 ± 0.39 | 0.125 ± 0.007 | 31.56 ± 1.59 | 0.53 ± 0.02 | 0.994 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 24.25 ± 0.27 | 0.207 ± 0.009 | 17.06 ± 0.62 | 0.42 ± 0.01 | 0.997 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 29.19 ± 5.17 | 0.042 ± 0.025 | 95.71 ± 64.35 | 0.39 ± 0.05 | 0.971 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 29.22 ± 4.42 | 0.056 ± 0.038 | 55.45 ± 40.32 | 0.31 ± 0.04 | 0.975 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 21.85 ± 2.50 | 0.089 ± 0.042 | 30.35 ± 14.03 | 0.36 ± 0.05 | 0.951 | <0.001 | |
| Esperase | T:−1 (37.3 °C)/pH:−1 (6.6) | NHD | NHD | NHD | NHD | NHD | NHD |
| T:1 (72.7 °C)/pH:−1 (6.6) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:−1 (37.3 °C)/pH:1 (9.4) | 12.95 ± 0.13 | 0.149 ± 0.007 | 24.13 ± 1.26 | 0.80 ± 0.05 | 0.972 | <0.001 | |
| T:1 (72.7 °C)/pH:1 (9.4) | 30.0 ± 18.04 | 0.004 (NS) | 604.8 (NS) | 0.25 ± 0.05 | 0.969 | <0.001 | |
| T:−1.41 (30.0 °C)/pH:0 (8.0) | 5.41 ± 0.08 | 0.041 ± 0.003 | 122.5 ± 2.62 | 2.66 ± 0.19 | 0.978 | <0.001 | |
| T:1.41 (80.0 °C)/pH:0 (8.0) | 11.73 ± 0.02 | 1.43 ± 0.06 | 2.10 ± 0.16 | 0.74 ± 0.05 | 0.981 | <0.001 | |
| T:0 (55.0 °C)/pH:−1.41 (6.0) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:0 (55.0 °C)/pH:1.41 (10.0) | 20.54 ± 0.05 | 0.532 ± 0.012 | 8.43 ± 0.29 | 0.63 ± 0.02 | 0.993 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 24.45 ± 0.18 | 0.249 ± 0.007 | 16.20 ± 0.40 | 0.48 ± 0.01 | 0.997 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 29.42 ± 0.54 | 0.209 ± 0.014 | 22.04 ± 0.93 | 0.45 ± 0.02 | 0.993 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 25.25 ± 0.23 | 0.281 ± 0.010 | 14.79 ± 0.47 | 0.48 ± 0.01 | 0.995 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 29.78 ± 0.29 | 0.318 ± 0.012 | 15.67 ± 0.52 | 0.48 ± 0.01 | 0.995 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 27.83 ± 0.21 | 0.322 ± 0.010 | 14.52 ± 0.40 | 0.49 ± 0.01 | 0.996 | <0.001 |
| Enzyme/Activity | Polynomial Equations | R2 | Fisher F-Test | Topt (°C) | pHopt | Ymax |
|---|---|---|---|---|---|---|
| Alcalase/Hydrolysis | Hm (%) = 25.22 + 3.97 T + 6.78 T pH − 4.82 T2 − 9.79 pH2 | 0.698 | F1: S; F2: S; F3: NS; F4: NS | 64.6 | 9.40 | 26.3% |
| vm (% min−1) = 0.104 + 0.092 T − 0.398 pH − 0.170 T pH − 0.088 T2 + 0.318 pH2 | 0.693 | F1: S; F2: S; F3: NS; F4: NS | 53.9 | 10.29 | 1.6% min−1 | |
| Esperase/Hydrolysis | Hm (%) = 27.34 + 3.25 T + 9.02 pH + 4.26 T pH − 9.09 T2 − 8.23 pH2 | 0.943 | F1: S; F2: S; F3: S; F4: S | 60.8 | 8.90 | 30.7% |
| vm (% min−1) = 0.277 + 0.228 T + 0.113 pH + 0.115 T2 − 0.122 pH2 | 0.413 | F1: S; F2: S; F3: NS; F4: NS | 80 | 8.65 | 0.90% min−1 | |
| Alcalase/Antihypertensive | IACE (%) = 74.51 + 7.61 T + 3.48 pH − 6.03 T2 − 6.50 pH2 | 0.746 | F1: S; F2: S; F3: NS; F4: NS | 66.2 | 11.5 | 90.7% |
| * IC50 (μg/mL) = 117.42 − 23.61 T + 47.81 T2 | 0.739 | F1: S; F2: S; F3: NS; F4: NS | 59.4 | non effect | 114.5 μg/mL | |
| Esperase/Antihypertensive | IACE (%) = 74.42 − 3.87 T | 0.496 | F1: S; F2: S; F3: NS; F4: NS | 30.0 | non effect | 79.9% |
| * IC50 (μg/mL) = 111.41 + 94.34 T + 80.12 T2 | 0.583 | F1: S; F2: S; F3: NS; F4: NS | 44.6 | non effect | 83.64 μg/mL |
| Enzyme | Polynomial Equations | R2 | Fisher F-Test | Topt (°C) | pHopt | Ymax |
|---|---|---|---|---|---|---|
| Alcalase | β-C (μg BHT/mL) = 0.08 + 0.28 pH − 0.09 T2 + 0.26 pH2 | 0.680 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 12.0 | 1.09 μg BHT/mL |
| Cr (μg Trolox/mL) = 1.63 − 0.95 T + 0.98 pH − 1.53 T pH | 0.543 | F1: S; F2: S; F3: NS; F4: NS | 30.0 | 12.0 | 7.96 μg Tr/mL | |
| DPPH (%) = 12.07 + 1.44 T − 1.57 T2 − 2.68 pH2 | 0.833 | F1: S; F2: S; F3: NS; F4: NS | 63.1 | 9.0 | 12.4% | |
| ABTS (%) = 5.10 + 1.24 T pH − 0.85 T2 − 0.82 pH2 | 0.578 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 9.0 | 5.1% | |
| Esperase | Cr (μg Trolox/mL) = 2.23 − 0.62 T2 − 0.61 pH2 | 0.625 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 2.23 μg Tr/mL |
| DPPH (%) = 16.02 − 2.31 T pH − 2.26 T2 − 2.49 pH2 | 0.840 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 16.0% | |
| ABTS (%) = 7.30 − 1.44 T2 − 1.64 pH2 | 0.752 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 7.3% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 306. https://doi.org/10.3390/md15100306
Vázquez JA, Blanco M, Massa AE, Amado IR, Pérez-Martín RI. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Marine Drugs. 2017; 15(10):306. https://doi.org/10.3390/md15100306
Chicago/Turabian StyleVázquez, José A., Maria Blanco, Agueda E. Massa, Isabel Rodríguez Amado, and Ricardo I. Pérez-Martín. 2017. "Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology" Marine Drugs 15, no. 10: 306. https://doi.org/10.3390/md15100306
APA StyleVázquez, J. A., Blanco, M., Massa, A. E., Amado, I. R., & Pérez-Martín, R. I. (2017). Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Marine Drugs, 15(10), 306. https://doi.org/10.3390/md15100306