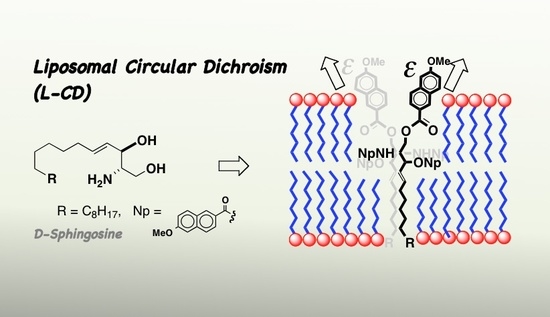
Liposomal Circular Dichroism (L-CD) of Arenoyl Derivatives of Sphingolipids. Amplification of Cotton Effects in Ordered Lipid Bilayers
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR Application of Mosher’s Method. The Absolute Configurations of Marine Terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Kusumi, T.; Hamada, T.; Ishitsuka, M.O.; Ohtani, I.; Kakisawa, H. Elucidation of the Relative and Absolute Stereochemistry of Lobatriene, a Marine Diterpene, by a Modified Mosher Method. J. Org. Chem. 1992, 57, 1033–1035. [Google Scholar] [CrossRef]
- Matsumori, N.; Kaneno, D.; Murata, M.; Nakamura, H.; Tachibana, K. Stereochemical Determination of Acyclic Structures Based on Carbon-Proton Spin-Coupling Constants. A Method of Configuration Analysis for Natural Products. J. Org. Chem. 1999, 64, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, P. Optical Rotatory Dispersion and Circular Dichroism in Organic Chemistry; Holden-Day: San Francisco, CA, USA, 1965. [Google Scholar]
- Lightner, D.A.; Gurst, G.E. Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Merten, C.; Smyrniotopoulos, V.; Tasdemir, D. Assignment of absolute configurations of highly flexible linear diterpenes from the brown alga Bifurcaria bifurcata by VCD spectroscopy. Chem. Commun. 2015, 51, 16217–16220. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, B.I.; Molinski, T.F. Xestoproxamines A-C from Neopetrosia proxima. Assignment of Absolute Stereostructure of bis-Piperidine Alkaloids by Integrated Degradation-CD Analysis. J. Nat. Prod. 2011, 74, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, B.I.; Skepper, C.K.; Molinski, T.F. Ene-yne Tetrahydrofurans from the Sponge Xestospongia muta. Exploiting a Weak CD Effect for Assignment of Configuration. Org. Lett. 2007, 9, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Wiesler, W.T. Pairwise Additivity in Circular Dichroism: Applications to Oligosaccharide and Acyclic Polyol Structural Analysis. Ph.D. Thesis, Columbia University, New York, NY, USA, 1989. [Google Scholar]
- Wiesler, W.T.; Nakanishi, K. A Simple Spectroscopic Method for Assigning Relative and Absolute Configuration in Acyclic 1,2,3-Triols. J. Am. Chem. Soc. 1989, 111, 3446–3447. [Google Scholar] [CrossRef]
- Wiesler, W.T.; Nakanishi, K. Relative and Absolute Configurational Assignments of Acyclic Polyols by Circular Dichroism. 2. Determination of Nondegenerate Exciton Coupling Interactions by Assignment of Prochiral Aryloxymethylene Protons for Proton NMR Conformational Analysis. J. Am. Chem. Soc. 1990, 112, 5574–5583. [Google Scholar] [CrossRef]
- Kázmierczak, F.; Gawronska, K.; Rychlewska, U.; Gawronski, J. Exciton Coupling of the Phthalimide Chromophore: Application to Configurational Assignments. Tetrahedron Asymmetry 1994, 5, 527–530. [Google Scholar] [CrossRef]
- Harada, N.; Nakanishi, K. Circular Dichroic Spectroscopy: Exciton Coupling in Organic Stereochemistry; University Science Books: Mill Valley, CA, USA, 1983; p. 460. [Google Scholar]
- Dirsch, V.; Frederico, J.; Zhao, N.; Cai, G.; Chen, Y.; Vunnam, S.; Odingo, J.; Pu, H.; Nakanishi, K.; Berova, N.; et al. A Two-Step Chemical and Circular Dichroic Method for Assigning the Absolute Configurations of Sphingosines. Tetrahedron Lett. 1995, 36, 4959–4962. [Google Scholar] [CrossRef]
- Kawamura, A.; Berova, N.; Dirsch, V.; Mangoni, A.; Nakanishi, K.; Schwartz, G.; Bielawska, A.; Hannun, Y.; Kitagawa, I. Picomole Scale Stereochemical Analysis of Sphingosine and Dihydrosphingosines. Bioorg. Med. Chem. Lett. 1996, 4, 1035–1043. [Google Scholar] [CrossRef]
- Munesada, K.; Yuasa, M.; Suga, T. Cerebrosides of Frog Brain. Structure of the Ceramide Part of the Cerebrosides. J. Chem. Soc. Perkin Trans. 1 1991, 0, 189–194. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Guzii, A.; Denisenko, V.A.; Dmitrenok, P.S.; Santalova, E.A.; Pokanevich, E.V.; Molinski, T.F.; Stonik, V.A. Rhizochalin A, a Novel Two-Headed Sphingolipid from the Sponge Rhizochalina incrustata. J. Nat. Prod. 2005, 68, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-N.; Mattern, M.P.; Johnson, R.K.; Kingston, D.G.I. Structure and Stereochemistry of a novel bioactive sphingolipid from a Calyx sp. Tetrahedron 2001, 57, 9549–9554. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Zakharenko, A.M.; Dmitrenok, P.S.; Guzii, A.G.; Denisenko, V.A.; Savina, A.S.; Dalisay, D.S.; Molinski, T.F.; Stonik, V.A. Isorhizochalin: A Minor Unprecedented Bipolar Sphingolipid of Stereodivergent Biogenesis from the Rhizochalina incrustata. Lipids 2009, 44, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Dmitrenok, P.S.; Zakharenko, A.M.; Denisenko, V.A.; Guzii, A.G.; Li, R.H.; Skepper, C.K.; Molinski, T.F.; Stonik, V.A. Rhizochalins C and D from the sponge Rhizochalina incrustata. A rare threo-sphingolipid and a facile method for determination of the carbonyl position in α,ω-bifunctionalized ketosphingolipids. J. Nat. Prod. 2007, 70, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hoff, J.H. Die Lagerung der Atome im Raume; Vieweg: Braunschweig, Germany, 1908; Volume 8, pp. 95–97. [Google Scholar]
- Molinski, T.F.; Makarieva, T.N.; Stonik, V.A. (–)-Rhizochalin is a Dimeric Enantiomorphic (2R)-Sphingolipid. Analysis of pseudo-C2v Symmetric bis-2-Amino-3-alkanols by CD. Angew. Chem. Int. Ed. 2000, 39, 4076–4079. [Google Scholar] [CrossRef]
- Nicholas, G.M.; Molinski, T.F. Enantiodivergent Biosynthesis of the Dimeric Sphingolipid Oceanapiside from the Marine Sponge Oceanapia phillipensis. Determination of Remote Stereochemistry. J. Am. Chem. Soc. 2000, 122, 4011–4019. [Google Scholar] [CrossRef]
- Nicholas, G.N.; Hong, T.W.; Molinski, T.F.; Lerch, M.L.; Cancilla, M.T.; Lebrilla, C.B. Oceanapiside, an Antifungal bis-α,ω-Aminoalcohol Glycoside from the Marine Sponge Oceanapia phillipensis. J. Nat. Prod. 1999, 62, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Tsukamoto, S.; Molinski, T.F. Absolute Configuration of the α,ω-Bifunctionalized Sphingolipid Leucettamol A from Leucetta sp. by Deconvoluted Exciton Coupled CD. J. Nat. Prod. 2009, 72, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kossuga, M.H.; MacMillan, J.B.; Rogers, E.W.; Molinski, T.F.; Nascimento, G.S.F.; Rocha, R.M.; Berlinck, R.G.S. (2S,3R)-2-Aminododecan-3-ol, a New Antifungal Agent from the Ascidian Clavelina oblonga. J. Nat. Prod. 2004, 67, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Biegelmeyer, R.; Stout, E.P.; Wang, X.; Frota, M.L.C.; Henriques, A.T. Halisphingosines A and B, Modified Sphingoid Bases from Haliclona tubifera. Assignment of Configuration by Circular Dichroism and van’t Hoff’s Principle of Optical Superposition. J. Nat. Prod. 2013, 76, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Fidder, H.; Knoester, J.; Wiersma, D.A. Observation of the one-exciton to two-exciton transition in a J aggregate. J. Chem. Phys. 1993, 98, 6564–6566. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The J- and H-bands of organic dye aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Molinski, T.F. Long-Range Stereo-Relay. Relative and Absolute Configuration of 1,n-Glycols from Circular Dichroism of Liposomal Porphyrin Esters. J. Am. Chem. Soc. 2004, 126, 9944–9945. [Google Scholar] [CrossRef] [PubMed]
- 1,2-Dilauroyl-sn-glycero-3-phosphocholine (DLPC, 12:0); 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, 14:0); 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, 16:0).; 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, 18:0).
- Dalisay, D.S.; Quach, T.; Molinski, T.F. Liposomal Circular Dichroism. Assignment of Remote Stereocenters in Plakinic Acids K and L from a Plakortis-Xestospongia Sponge Association. Org. Lett. 2010, 12, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Quach, T.; Nicholas, G.N.; Molinski, T.F. Amplification of Cotton Effects of a Single Chromophore through Liposomal Ordering. Stereochemical Assignment of Plakinic Acids (I-J) from Plakortis. Angew. Chem. Int. Ed. 2009, 48, 4367–4371. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Fuentes, P.; Alonso-Gómez, J.-L.; Petrovic, A.G.; Santoro, F.; Harada, N.; Berova, N.; Diederich, F. Amplification of Chirality in Monodisperse, Enantiopure Alleno-Acetylenic Oligomers. Angew. Chem. Int. Ed. 2010, 49, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Berg, C.J.; Hsu, C.-C.; Merrill, B.A.; Tauber, M.J. Characterization of Carotenoid Aggregates by Steady-State Optical Spectroscopy. J. Phys. Chem. B 2012, 116, 10617–10630. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Rogers, E.W.; Edison, A.; Molinski, T.F. Structure Elucidation at Nanomole-Scale. 1. Trisoxazole macrolides and Thiazole-containing Cyclic Peptides from the Nudibranch Hexabranchus sanguineus. J. Nat. Prod. 2009, 72, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, N.; Lo, L.-C.; Nakanishi, K. Detection of Subpicomole Levels of Compounds Containing Hydroxyl and Amino Groups with the Fluorogenic Reagent, 2-Naphthoylimidazole. Angew. Chem. Int. Ed. 1992, 31, 890–891. [Google Scholar] [CrossRef]
- Nehira, T.; Parish, C.A.; Jockusch, S.; Turro, N.J.; Nakanishi, K.; Berova, N. Fluorescence-Detected Exciton-Coupled Circular Dichroism: Scope and Limitation in Structural Studies of Organic Molecules. J. Am. Chem. Soc. 1999, 121, 8681–8691. [Google Scholar] [CrossRef]





| Entry | Cmpd. | MeOH | L-CD | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | +8.2 (224) | −2.2 (240) | −41.5 (210) | +28.0 (241) | −36.0 (255) | - | - |
| 2 | 7 | +6.3 (220) | −10.1 (238) | −14 (204) | +17.6 (216) | −13.0 (227) | +33.3 (242) | −12.6 (253) |
| 3 | 8 | +71.2 (232) | −43.7 (255) | +114 (228) | −44.5 (249) | −31.5 (266) | −14.5 (303) | - |
| 4 | 10 | +52.2 (231) | −45.2 (255) | +227 (230) | −104 (249) | −80 (262) | −17.4 (301) | - |
| 5 | 11 | 1 | 1 | 1 | 1 | - | - | - |
| 6 | 12 | 1 | 1 | 1 | 1 | - | - | - |
| 7 | (S)-14 2 | 1 | 1 | −23.8 (213) 2 | +34.6 (233) 2 | - | - | - |
| 8 | (±)-14 2 | 1 | 1 | 1 | 1 | - | - | - |
| 9 | 15 2 | 1 | 1 | 1 | 1 | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinski, T.F.; Broaddus, C.D.; Morinaka, B.I. Liposomal Circular Dichroism (L-CD) of Arenoyl Derivatives of Sphingolipids. Amplification of Cotton Effects in Ordered Lipid Bilayers. Mar. Drugs 2017, 15, 352. https://doi.org/10.3390/md15120352
Molinski TF, Broaddus CD, Morinaka BI. Liposomal Circular Dichroism (L-CD) of Arenoyl Derivatives of Sphingolipids. Amplification of Cotton Effects in Ordered Lipid Bilayers. Marine Drugs. 2017; 15(12):352. https://doi.org/10.3390/md15120352
Chicago/Turabian StyleMolinski, Tadeusz F., Caroline D. Broaddus, and Brandon I. Morinaka. 2017. "Liposomal Circular Dichroism (L-CD) of Arenoyl Derivatives of Sphingolipids. Amplification of Cotton Effects in Ordered Lipid Bilayers" Marine Drugs 15, no. 12: 352. https://doi.org/10.3390/md15120352
APA StyleMolinski, T. F., Broaddus, C. D., & Morinaka, B. I. (2017). Liposomal Circular Dichroism (L-CD) of Arenoyl Derivatives of Sphingolipids. Amplification of Cotton Effects in Ordered Lipid Bilayers. Marine Drugs, 15(12), 352. https://doi.org/10.3390/md15120352