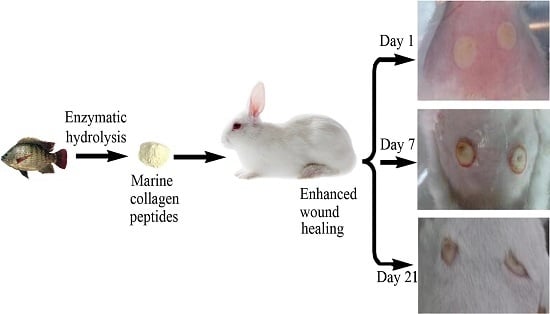
Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Weight Distribution of MCPs
2.2. Amino Acid Composition of MCPs
2.3. FTIR Analysis
2.4. Effect of MCPs on the Scratch Closure In Vitro
2.5. Wound Healing In Vivo
2.5.1. Scald Model Establishment
2.5.2. Effects of MCPs on Scald Wound Healing Rate
2.5.3. Histological Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of MCPs from the Skin of Tilapia
3.3. Determination of Molecular Weight Distribution of MCPs
3.4. Amino Acid Composition Measurement of MCPs
3.5. FTIR Analysis
3.6. In Vitro Scratch Assay
3.7. Effects of MCPs on Skin Scald Wound Healing in Rabbits
3.7.1. Establishment of the Animal Model
3.7.2. Grouping and Treatment
3.7.3. Determination of Wound Healing Rate
3.7.4. Histological Examination
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edelman, L.S. Social and economic factors associated with the risk of burn injury. Burns 2007, 33, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xu, S.; Ma, L.; Huang, A.; Gao, C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials 2011, 32, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, N.; Uma, T.S.; Lakshmi, T.S.R.; Babu, M. Efficiency of controlled topical delivery of silver sulfadiazine in infected burn wounds. J. Biomed. Mater. Res. A 2008, 89, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Richard, R. Partial-thickness burns: Identification and management. Adv. Skin Wound Care 2003, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Muthumari, K.; Anand, M.; Maruthupandy, M. Collagen extract from marine finfish scales as a potential mosquito larvicide. Protein J. 2016, 35, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Silva, J.; Silva, T.H.; Prata, M.B.; Cerqueira, M.T.; Pirraco, R.P.; Giovine, M.; Marques, A.P.; Reis, R.L. Potential of marine sponge collagen coatings for skin regeneration strategies. J. Tissue Eng. Regen. Med. 2013, 7, 33. [Google Scholar]
- Shen, X.R.; Kurihara, H.; Takahashi, K. Characterization of molecular species of collagen in scallop mantle. Food Chem. 2007, 102, 1187–1191. [Google Scholar]
- Mizuta, S.; Tanaka, T.; Yoshinaka, R. Comparison of collagen types of arm and mantle muscles of the common octopus (Octopus vulgaris). Food Chem. 2003, 81, 527–532. [Google Scholar] [CrossRef]
- Su, X.R.; Sun, B.; Li, Y.Y.; Hu, Q.H. Characterization of acid-soluble collagen from the coelomic wall of Sipunculida. Food Hydrocoll. 2009, 23, 2190–2194. [Google Scholar] [CrossRef]
- Kolodziejska, I.; Sikorski, Z.E.; Niecikowska, C. Parameters affecting the isolation of collagen from squid (Illex argentinus) skins. Food Chem. 1999, 66, 153–157. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishnaraj, M.; Prabhasankar, P. Marine protein hydrolysates: Their present and future perspectives in food chemistry—A review. RSC Adv. 2015, 5, 34864–34877. [Google Scholar] [CrossRef]
- Fan, L.; Cao, M.; Gao, S.; Wang, T.; Wu, H.; Peng, M.; Zhou, X.; Nie, M. Preparation and characterization of sodium alginate modified with collagen peptides. Carbohydr. Polym. 2013, 93, 380–385. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of Croceine Croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Ngo, D.H.; Vo, T.S. Marine fish-derived bioactive peptides as potential antihypertensive agents. Adv. Food Nutr. Res. 2012, 65, 249–260. [Google Scholar] [PubMed]
- Zhang, F.; Wang, Z.; Xu, S. Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability. Food Chem. 2009, 117, 387–392. [Google Scholar] [CrossRef]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Effect of marine collagen peptides on markers of metabolic nuclear receptors in type 2 diabetic patients with/without hypertension. Biomed. Environ. Sci. 2010, 23, 113–120. [Google Scholar] [CrossRef]
- Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of marine collagen peptides on physiological and neurobehavioral development of male rats with perinatal asphyxia. Mar. Drugs 2015, 13, 3653–3671. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B Biointerfaces 2016, 143, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.K.; Zhang, C.H.; Lin, H.; Yang, P.; Hong, P.Z.; Jiang, Z. Isolation and characterisation of acid-solubilised collagen from the skin of Nile tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chai, Y.; Wang, Q.; Liu, H.; Wang, S.; Xiao, J. A natural interruption displays higher global stability and local conformational flexibility than a similar Gly mutation sequence in collagen mimic peptides. Biochemistry 2015, 54, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Haris, P.I.; Severcan, F. FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal. B Enzym. 1999, 7, 207–221. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, M.; Yi, H.; Wang, J. Biosynthesis and characterization of a non-repetitive polypeptidederived from silk fibroin heavy chain. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mu, L.; Tang, J.; Shen, C.; Gao, C.; Rong, M.; Zhang, Z.; Liu, J.; Wu, X.; Yu, H.; et al. A potential wound healing-promoting peptide from frog skin. Int. J. Biochem. Cell Biol. 2014, 49, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.H.; Nguyen, T.T.T.; Park, J.S.; Hwang, H.M.; Song, M.S.; Lee, D.; Chai, K.Y. Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules 2012, 17, 2992–3007. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santonia, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin Pharmacol. Phys. 2014, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Y.; Shu, B.; Xie, X.; Yang, R.; Zhang, L.; Ruan, S.; Lin, Y.; Lin, Z.; Shen, R.; et al. The effect of porcine ADM to improve the burn wound healing. Int. J. Clin. Exp. Pathol. 2013, 6, 2280–2291. [Google Scholar] [PubMed]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [PubMed]
- Nagelschmidt, M.; Becker, D.; Bönninghoff, N.; Engelhardt, G.H. Effect of fibronectin therapy and fibronectin deficiency on wound healing: A study in rats. J. Trauma 1987, 27, 1267–1271. [Google Scholar] [CrossRef] [PubMed]





| Amino Acids | Contains (g/100 g) | Residues Per 1000 Total Amino Acid Residues |
|---|---|---|
| Aspartic acid | 5.53 | 48 |
| Threonine * | 2.67 | 25 |
| Serine | 3.17 | 34 |
| Glutamic acid | 9.40 | 81 |
| Glycine | 20.92 | 317 |
| Alanine | 9.23 | 118 |
| Valine * | 2.17 | 22 |
| Methionine * | 1.33 | 10 |
| Isoleucine * | 1.33 | 11 |
| Leucine * | 3.18 | 27 |
| Tyrosine | 0.74 | 6 |
| Phenylalanine * | 2.17 | 15 |
| Histidine | 1.01 | 8 |
| Lysine * | 3.33 | 26 |
| Arginine | 7.96 | 52 |
| Proline | 11.32 | 111 |
| Hydroxy proline | 10.28 | 89 |
| Total | 95.74 | 1000 |
| Post-Scald Day | Model Control Group | Positive Control Group | MCPs Group |
|---|---|---|---|
| 3 | −16.4 ± 19.3 | −22.7 ± 22.9 | −11.8 ± 23.1 |
| 7 | −7.0 ± 23.1 | −1.8 ± 27.5 | −3.6 ± 28.6 |
| 11 | 8.7 ± 17.2 | 19.5 ± 35.0 | 38.8 ± 22.8 **,# |
| 14 | 56.6 ± 31.1 | 70.5 ± 23.5 | 78.6 ± 11.1 * |
| 18 | 72.1 ± 13.9 | 95.3 ± 6.4 ** | 95.9 ± 7.2 ** |
| 21 | 86.2 ± 16.0 | 98.9 ± 2.0 ** | 98.0 ± 6.8 ** |
| 24 | 89.8 ± 6.3 | 100.0 ± 0 ** | 100.0 ± 0 ** |
| 28 | 100.0 ± 0 | 100.0 ± 0 | 100.0 ± 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. https://doi.org/10.3390/md15040102
Hu Z, Yang P, Zhou C, Li S, Hong P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Marine Drugs. 2017; 15(4):102. https://doi.org/10.3390/md15040102
Chicago/Turabian StyleHu, Zhang, Ping Yang, Chunxia Zhou, Sidong Li, and Pengzhi Hong. 2017. "Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation" Marine Drugs 15, no. 4: 102. https://doi.org/10.3390/md15040102
APA StyleHu, Z., Yang, P., Zhou, C., Li, S., & Hong, P. (2017). Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Marine Drugs, 15(4), 102. https://doi.org/10.3390/md15040102