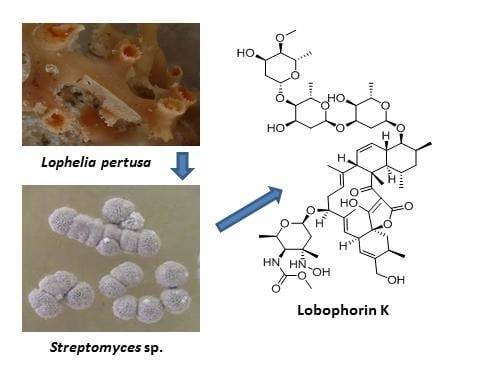
Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa
Abstract
:1. Introduction
2. Results
2.1. Taxonomy and Phylogenetic Analysis of the Strain M-207
2.2. Structure Determination
2.3. Antimicrobial Activity of Lobophorin K
2.4. Cytotoxic Activity of Lobophorin K
3. Experimental Section
3.1. General Experimental Procedures
3.2. Phylogenetic Analysis of the Producing Microorganism
3.3. Microorganism and Fermentation Conditions
3.4. Isolation and Purification of Lobophorin K
3.5. Antimicrobial Activity of Lobophorin K
3.6. Cytotoxic Activity of Lobophorin K
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, Z.D.; Jensen, P.R.; Fenical, W. Lobophorins A and B, new antiinflammatory macrolides produced by a tropical marine bacterium. Bioorg. Med. Chem. Lett. 1999, 19, 2003–2006. [Google Scholar] [CrossRef]
- Niu, S.; Li, S.; Chen, Y.; Tian, X.; Zhang, H.; Zhang, G.; Zhang, W.; Yang, X.; Zhang, S.; Ju, J.; et al. Lobophorins E and F, new spirotetronate antibiotics from a South China Sea-derived Streptomyces sp. SCSIO 01127. J. Antibiot. 2011, 64, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Guo, H.; Hou, W.; Yang, N.; Ren, B.; Liu, M.; Dai, H.; Liu, X.; Song, F.; et al. Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061. Appl. Microbiol. Biotechnol. 2013, 97, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Q.; Zhang, S.Y.; Wang, N.; Li, Z.L.; Hua, H.M.; Hu, J.C.; Wang, S.J. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar. Drugs 2013, 11, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Koch, M.; Pond, C.D.; Mabeza, G.; Seronay, R.A.; Concepcion, G.P.; Barrows, L.R.; Olivera, B.M.; Schmidt, E.W. Structure and activity of lobophorins from a turrid mollusk-associated Streptomyces sp. J. Antibiot. 2014, 67, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.G.; Fribley, A.M.; Miller, J.R.; Larsen, M.J.; Schultz, P.J.; Jacob, R.T.; Tamayo-Castillo, G.; Kaufman, R.J.; Sherman, D.H. Novel Lobophorins Inhibit Oral Cancer Cell Growth and Induce Atf4- and Chop-Dependent Cell Death in Murine Fibroblasts. ACS Med. Chem. Lett. 2015, 6, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-F.; Pan, H.-Q.; Hu, J.-C. Isolation and identification of a new antibiotic, lobophorin J, from a deep sea-derived Streptomyces sp. 12A35. Chin. J. Antibiot. 2015, 40, 721–727. [Google Scholar]
- Wei, R.-B.; Xi, T.; Li, J.; Wang, P.; Li, F.-C.; Lin, Y.-C.; Qin, S. Lobophorin C and D, new kijanimicin derivatives from a marine sponge-associated actinomycetal strain AZS17. Mar. Drugs 2011, 9, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Braña, A.F.; Fiedler, H.-P.; Nava, H.; González, V.; Sarmiento-Vizcaíno, A.; Molina, A.; Acuña, J.L.; García, L.A.; Blanco, G. Two Streptomyces Species Producing Antibiotic, Antitumor, and Anti Inflammatory Compounds Are Widespread Among Intertidal Macroalgae and Deep Sea Coral Reef Invertebrates from the Central Cantabrian Sea. Microb. Ecol. 2015, 69, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Molina, A.; Acuña, J.L.; García, L.A.; Blanco, G. Myceligenerans cantabricum sp. nov., a barotolerant actinobacterium isolated from a deep cold water coral. Int. J. Syst. Evol. Microbiol. 2015, 65, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; Braña, A.F.; González, V.; Nava, H.; Molina, A.; Llera, E.; Fiedler, H.P.; Rico, J.M.; García-Flórez, L.; Acuña, J.L.; et al. Atmospheric Dispersal of Bioactive Streptomyces albidoflavus Strains Among Terrestrial and Marine Environments. Microb. Ecol. 2016, 71, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Palacios, J.J.; Otero, L.; Fernández, J.; Molina, A.; Kulik, A.; Vázquez, F.; Acuña, J.L.; et al. Pharmacological potential of phylogenetically diverse Actinobacteria isolated from deep-sea coral ecosystems of the submarine Avilés Canyon in the Cantabrian Sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.G.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Conference limits on phylogenies: an approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Braña, A.F.; Rodríguez, M.; Pahari., P.; Rohr, J.; García, L.A.; Blanco, G. Activation and silencing of secondary metabolites in Streptomyces albus and Streptomyces lividans after transformation with cosmids containing the thienamycin gene cluster from Streptomyces cattleya. Arch. Microbiol. 2014, 196, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Audoin, C.; Bonhomme, D.; Ivanisevic, J.; de la Cruz, M.; Cautain, B.; Monteiro, M.C.; Reyes, F.; Rios, L.; Perez, T.; Thomas, O.P. Balibalosides, an Original Family of Glucosylated Sesterterpenes Produced by the Mediterranean Sponge Oscarella balibaloi. Mar. Drugs 2013, 11, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R.J. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]


| Position | δ 1H (mult, J, Hz) | Δ 13C | Position | δ 1H (mult, J, Hz) | δ 13C |
|---|---|---|---|---|---|
| 1 | - | n.d. | - | - | - |
| 2 | - | 100.7 | A1 | 4.77 (br d, 4.1) | 99.7 |
| 3 | - | 205.0 a | A2 | 2.39 (m), 1.74 (m) | 31.0 |
| 4 | - | 52.4 | A3 | 4.02 (dd, 6.1, 2.9) | 69.3 |
| 5 | 2.08 (m) | 44.8 | A4 | 3.26 (dd, 9.6, 3.3) | 73.4 |
| 6 | 1.61 (m) | 32.5 | A5 | 4.10 (m) | 66.0 |
| 7 | 1.61 (m), 1.53 (m) | 43.0 | A6 | 1.21 (d, 6.4) | 18.2 |
| 8 | 2.24 (m) | 35.9 | - | - | - |
| 9 | 3.42 (dd, 10.1, 5.3) | 86.2 | B1 | 5.17 (br d, 3.5) | 93.2 |
| 10 | 2.09 (m) | 39.7 | B2 | 2.09 (m), 1.99 (dt, 14.6, 3.7) | 35.9 |
| 11 | 5.81 (br d, 10.2) | 127.2 | B3 | 4.18 (dd, 6.5, 3.5) | 68.1 |
| 12 | 5.38 (ddd, 10.2, 4.9, 1.8) | 127.9 | B4 | 3.29 (m) | 83.3 |
| 13 | 3.65 (m) | 52.7 | B5 | 4.06 (m) | 63.7 |
| 14 | - | 137.1 | B6 | 1.21 (d, 6.3) | 18.0 |
| 15 | 5.20 (brd, 9.4) | 124.9 | - | - | - |
| 16 | 2.40 (m), 2.26 (m) | 32.5 | C1 | 4.95 (dd, 9.7, 1.7) | 100.7 |
| 17 | 4.20 (m) | 80.3 | C2 | 2.05 (m), 1.72 (m) | 38.8 |
| 18 | - | 139.3 | C3 | 4.29 (dd, 5.8, 2.8) | 64.4 |
| 19 | 5.12 (br d 10.5) | 120.2 | C4 | 2.85 (dd, 9.4, 2.8) | 83.8 |
| 20 | 3.59 (br d, 10.4) | 41.5 | C5 | 3.81 (dq, 9.4, 6.2) | 69.6 |
| 21 | 5.43 (br s) | 122.7 | C6 | 1.23 (d, 6.3) | 18.6 |
| 22 | - | 142.5 | C7 | 3.38 (s) | 57.0 |
| 23 | 2.62 (m) | 29.0 | - | - | - |
| 24 | 2.39 (m), 1.79 (d, 14.2) | 36.4 | D1 | 4.73 (dd, 9.8, 2.5) | 99.2 |
| 25 | - | 84.9 | D2 | 1.61 (m), 1.50 (dd, 14.3, 9.8) | 37.5 |
| 26 | - | 200.9 | D3 | - | 61.6 |
| 27 | 1.55 (s) | 15.5 | D4 | 3.66 (m) | 54.1 |
| 28 | 0.65 (br d, 4.2) | 22.9 | D5 | 4.23 (qd, 6.4, 1.6) | 69.4 |
| 29 | 1.14 (d, 7.0) | 14.8 | D6 | 1.08 (d, 6.4) | 17.5 |
| 30 | 1.38 (br s) | 14.2 | D7 | 1.15 (s) | 22.6 |
| 31 | 1.41 (br s) | 15.3 | D8 | - | 160.1 |
| 32 | 4.14 (br s), 4.09 (m) | 65.0 | D9 | 3.65 (s) | 52.4 |
| 33 | 1.28 (d, 7.2) | 20.5 | - | - | - |
| Bacteria | Lobophorin K (MIC90 µg/mL) |
|---|---|
| Gram-positive | - |
| Staphylococcus aureus EPI1167 MSSA | 40–80 |
| Staphylococcus aureus MB5393 MRSA | >160 |
| Gram-negative | - |
| Acinetobacter baumannii MB5973 | >160 |
| Pseudomonas aeruginosa PAO1 | >160 |
| Klebsiella pneumoniae ATCC 700603 | >160 |
| Escherichia coli MB2884 | >160 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braña, A.F.; Sarmiento-Vizcaíno, A.; Osset, M.; Pérez-Victoria, I.; Martín, J.; De Pedro, N.; De la Cruz, M.; Díaz, C.; Vicente, F.; Reyes, F.; et al. Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Mar. Drugs 2017, 15, 144. https://doi.org/10.3390/md15050144
Braña AF, Sarmiento-Vizcaíno A, Osset M, Pérez-Victoria I, Martín J, De Pedro N, De la Cruz M, Díaz C, Vicente F, Reyes F, et al. Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Marine Drugs. 2017; 15(5):144. https://doi.org/10.3390/md15050144
Chicago/Turabian StyleBraña, Alfredo F., Aida Sarmiento-Vizcaíno, Miguel Osset, Ignacio Pérez-Victoria, Jesús Martín, Nuria De Pedro, Mercedes De la Cruz, Caridad Díaz, Francisca Vicente, Fernando Reyes, and et al. 2017. "Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa" Marine Drugs 15, no. 5: 144. https://doi.org/10.3390/md15050144
APA StyleBraña, A. F., Sarmiento-Vizcaíno, A., Osset, M., Pérez-Victoria, I., Martín, J., De Pedro, N., De la Cruz, M., Díaz, C., Vicente, F., Reyes, F., García, L. A., & Blanco, G. (2017). Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Marine Drugs, 15(5), 144. https://doi.org/10.3390/md15050144