The Missing Piece in Biosynthesis of Amphidinols: First Evidence of Glycolate as a Starter Unit in New Polyketides from Amphidinium carterae
Abstract
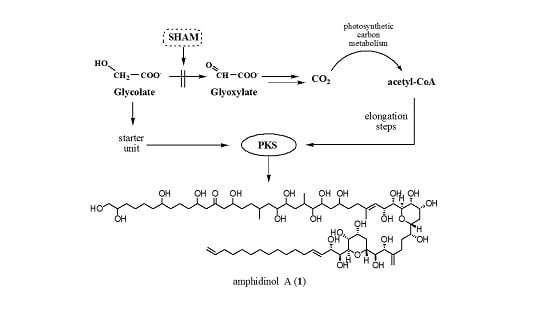
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation of Amphidinols 1 and 2
3.4. Biosynthetic Experiments
3.4.1. Control
3.4.2. Feeding Experiments with Labelled Acetate
Experiments with [1-13C]-Acetate
Experiments with [2-13C]-Acetate
Experiments with [1,2-13C2]-Acetate
3.4.3. Feeding Experiments with Labelled Glycolate
Experiments with [1-13C]-Glycolate
Experiments with [1-13C]-Glycolate/Salicylhydroxamic Acid (SHAM)
3.5. Antifungal Test
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainible source of bioactive molecules. Mar. Environ. Res. 2016. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and application of a novel SPE-method for bioassay-guided fractionation of marine extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a polyhydroxypolyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Paul, G.K.; Matsumori, N.; Murata, M.; Tachibana, K. Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetrahedron Lett. 1995, 36, 6279–6282. [Google Scholar]
- Paul, G.K.; Matsumori, N.; Konoki, K.; Sasaki, M.; Murata, M.; Tachibana, K. Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Tukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 503–506. [Google Scholar]
- Paul, G.K.; Matsumori, N.; Konoki, K.; Murata, M.; Tachibana, K. Chemical structures of amphidinols 5 and 6 isolated from marine dinoflagellate Amphidinium klebsii and their cholesterol-dependent membrane disruption. J. Mar. Biotechnol. 1997, 5, 124–128. [Google Scholar]
- Houdai, T.; Matsuoka, S.; Murata, M.; Satake, M.; Ota, S.; Oshima, Y.; Rhodes, L.L. Acetate labeling patterns of dinoflagellate polyketides, amphidinols 2, 3 and 4. Tetrahedron 2001, 57, 5551–5555. [Google Scholar] [CrossRef]
- Morsy, N.; Matsuoka, S.; Houndai, T.; Matsumori, N.; Adachi, S.; Murata, M.; Iwashita, T.; Fujita, T. Isolation and structure elucidation of a new amphidinol with truncated polyhydroxyl chain from Amphidinium klebsii. Tetrahedron 2005, 61, 8606–8610. [Google Scholar] [CrossRef]
- Echigoya, R.; Rhodes, L.; Oshima, Y.; Satake, M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae 2005, 4, 383–389. [Google Scholar] [CrossRef]
- Morsy, N.; Houdai, T.; Matsuoka, S.; Matsumori, N.; Adachi, S.; Oishi, T.; Murata, M.; Iwashita, T.; Fujita, T. Structures of new amphidinols with truncated polyhydroxyl chain and their membrane-permeabilizing activity. Bioorg. Med. Chem. 2006, 14, 6548–6554. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Van Wagoner, R.M.; Misner, I.; Tomas, C.; Wright, J.L.C. Structure and biosynthesis of amphidinol 17, a hemolytic compound from Amphidinium carterae. J. Nat. Prod. 2010, 73, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kuo, C.; Lin, Y.; Chen, Y.; Lu, C.; Carteraol, E. a potent polyhydroxyl ichthyotoxin from the dinoflagellate Amphidinium carterae. Tetrahedron Lett. 2009, 50, 2512–2515. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, D.; Guo, Y.; Wu, H.; Lin, L.; Wang, Z.; Ding, J.; Lin, Y. Lingshuiol, a novel polyhydroxyl compound with strongly cytotoxic activity from the marine dinoflagellate Amphidinium sp. Biorg. Med. Chem. Lett. 2004, 14, 3117–3120. [Google Scholar]
- Huang, X.; Zhao, D.; Guo, Y.; Wu, H.; Trivellone, E.; Cimino, G. Lingshuiols A and B, two new polyhydroxy compounds from the Chinese marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2004, 45, 5501–5504. [Google Scholar] [CrossRef]
- Hanif, N.; Ohno, O.; Kitamura, M.; Yamada, K.; Uemura, D. Symbiopolyol, a VCAM-1 inhibitor from a symbiotic dinoflagellate of the jellyfish Mastigias papua. J. Nat. Prod 2010, 73, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Washisa, K.; Koyama, T.; Yamada, K.; Kita, M.; Uemura, D. Karatungiols A and B, two novel antimicrobial polyol compounds, from the symbiotic marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006, 47, 2521–2525. [Google Scholar] [CrossRef]
- Van Wagoner, R.; Deeds, J.R.; Satake, M.; Ribeiro, A.A.; Place, A.R.; Wright, J.L.C. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 2008, 49, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Place, A.R.; Yoshida, W.; Anklin, C.; Hamann, M.T. Structure and absolute configuration of karlotoxin-2, an ichthyotoxin from the marine dinoflagellate Karlodinium veneficum. J. Am. Chem. Soc. 2010, 132, 3277–3279. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Takahashi, A.; Tsuda, M.; Kobayashi, J. Luteophanol D, new polyhydroxyl metabolite from marine dinoflagellate Amphidinium sp. Mar. Drugs 2005, 3, 113–118. [Google Scholar] [CrossRef]
- Inuzuka, T.; Yamamoto, Y.; Yamada, K.; Uemura, D. Amdigenol A, a long carbon-backbone polyol compound, produced by the marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2012, 53, 239–242. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Swasono, R.T.; Mouri, R.; Morsy, N.; Matsumori, N.; Oishi, T.; Murata, M. Sterol effect on interaction between amphidinol 3 and liposomal membrane as evidenced by surface plasmon resonance. Bioorg Med. Chem. Lett. 2010, 20, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Houdai, T.; Matsuoka, S.; Morsy, N.; Matsumori, N.; Satake, M.; Murata, M. Hairpin conformation of amphidinols possibly accounting for potent membrane permeabilizing activities. Tetrahedron 2005, 61, 2795–2802. [Google Scholar] [CrossRef]
- Needham, J.; McLachlan, J.L.; Walter, J.A.; Wright, J.L. Biosynthetic origin of C-37 and C-38 in the polyether toxins okadaic acid and dinophysistoxin-1. J. Chem. Soc. Chem. Comm. 1994, 22, 2599–2600. [Google Scholar] [CrossRef]
- Kellman, R.; Stüken, A.; Orr, R.J.S.; Svendsen, H.M.; Jakobsen, K.S. Biosynthesis and molecular genetics of polyketides in marine dinoflagellates. Mar. Drugs 2010, 8, 1011–1048. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Kalaitzis, J.A.; Moore, B.S. EncM, a versatile enterocyn biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15609–15614. [Google Scholar] [CrossRef] [PubMed]
- Julien, B.; Tian, Z.Q.; Reid, R.; Reeves, C.D. Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanism of polyketide biosynthesis. Chem. Biol. 2006, 13, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.J.; Holker, J.S.E. The biosynthesis of a pyrone metabolite of Aspergillus melleus. An application of long-range 13C-13C coupling constants. Tetrahedron Lett. 1975, 16, 4693–4696. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Hu, T.; McLachlan, J.L.; Needham, J.; Walter, J.A. Confirmation of a polyketide pathway, proof of a Bayer-Villiger oxidation step, and evidence for an unusual carbon deletion process. J. Am. Chem. Soc. 1996, 118, 8757–8758. [Google Scholar] [CrossRef]
- Kisaki, T.; Tolbert, N.E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tolbert, N.E. Association of glycolate oxidation with photosynthetic electron transport in plant and algal chloroplasts. Proc. Natl. Acad. Sci. USA 1996, 93, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A. Glycolate metabolism in algal chloroplasts: Inhibition by salicylhydroxamic acid (SHAM). Physiol. Plant. 2002, 116, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Selvin, C.R.; Claus, W.; Guillard, R.R. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]






| Position | Type | 1 | 2 | Position | Type | 1 | 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3OD/C5N5D (2:1) | CD3OD | CD3OD/C5N5D (2:1) | CD3OD | ||||||||
| δC | δH, mult, J (Hz) | Type | δH, mult, J (Hz) | δC | δH, mult, J (Hz) | δC | δH, mult, J (Hz) | ||||
| 1 | CH2 | 67.6 | 3.55, m; 3.59, m | 67.1 | 3.44, dd, 11.0, 6.3; 3.51, m | 33 | CH | 72.7 | 3.87, dd, 9.0, 2.0 | 72.4 | 3.70, m |
| 2 | CH | 73.1 | 3.71, m | 73.0 | 3.61, m | 34 | CH | 79.2 | 4.26, dd, 9.5, 2.0 | 79.1 | 3.99 d, m |
| 3 | CH2 | 34.7 | 1.47, m; 1.57, m | 34.2 | 1.41, m | 35 | CH | 69.0 | 4.34, m | 67.2 | 4.07, m |
| 4 | CH2 | 26.8 | 1.40, m; 1.46, m | 26.6 | 1.43,m; 1.52, m | 36 | CH | 67.2 | 4.16, m | 67.2 | 4.00, m |
| 5 | CH2 | 26.9 | 1.47, m; 1.55, m | 25.8 | 1.48, m | 37 | CH2 | 30.7 | 1.98, dt, 12.0, 3.6; 2.05, q, 12.0 | 30.2 | 1.82, m |
| 6 | CH2 | 38.6 | 1.46, m; 1.52, m | 35.2 | 1.50, m; 1.70, m | 38 | CH | 75.8 | 3.65 b, m | 75.5 | 3.55 c, m |
| 7 | CH | 71.8 | 3.58, m | 80.3 | 4.39 a, m | 39 | CH | 74.5 | 3.75, m | 74.0 | 3.64, m |
| 8 | CH2 | 38.6 | 1.46, m; 1.52, m | 35.3 | 1.50, m; 1.70, m | 40 | CH2 | 32.6 | 1.73, m; 2.12, m | 32.1 | 1.60, m; 2.0, m |
| 9 | CH2 | 22.9 | 1.45, m; 1.70, m | 21.9 | 1.49, m; 1.60, m | 41 | CH2 | 27.9 | 2.27, m | 27.7 | 2.15, m; 2.45, m |
| 10 | CH2 | 38.6 | 1.46, m; 1.52, m | 38.4 | 1.48, m; 1.55, m | 42 | C | 152.0 | - | 151.3 | - |
| 11 | CH | 68.6 | 4.18, m | 68.6 | 4.09, m | 43 | CH | 76.8 | 4.43, d, 8.6 | 76.4 | 4.22, d, 8.8 |
| 12, 14 | CH2 | 51.9 | 2.69, m | 51.6 | 2.65, m | 44 | CH | 75.3 | 3.54, m | 75.1 | 3.38, m |
| 13 | C | 211.2 | - | 211.6 | - | 45 | CH | 70.6 | 4.25, dt, 12.0, 2.0 | 70.4 | 4.07, m |
| 15 | CH | 68.9 | 4.13, m | 68.8 | 4.07, m | 46 | CH2 | 31.9 | 1.68, m; 2.36, q, 12.0 | 31.5 | 1.60, m; 2.12, m |
| 16 | CH2 | 35.7 | 1.49, m; 1.59, m | 35.5 | 1.40, m; 1.50, m | 47 | CH | 67.3 | 4.21, dd, 10.0, 3.5 | 69.2 | 4.07 e, m |
| 17 | CH2 | 33.1 | 1.14, m;1.72, m | 33.0 | 1.15, m; 1.62, m | 48 | CH | 68.6 | 4.33, m | 68.6 | 4.07 e, m |
| 18 | CH2 | 30.6 | 1.76, m | 30.2 | 1.70, m | 49 | CH | 80.7 | 4.02, dd, 10.0, 1.5 | 80.4 | 3.78 m |
| 19 | CH2 | 41.3 | 1.47, m; 1.55, m | 41.8 | 1.40, m; 1.50, m | 50 | CH | 72.0 | 4.22, m | 71.9 | 3.99 d, m |
| 20 | CH | 73.3 | 3.65 b, m | 73.3 | 3.55 c, m | 51 | CH | 74.2 | 4.62, dd, 10.0, 1.5 | 74.1 | 4.39 a, m |
| 21 | CH | 72.6 | 3.69, m | 72.6 | 3.5, m | 52 | CH | 129.1 | 5.79, dd, 15.5, 7.0 | 128.1 | 5.63, dd, 15.0, 7.5 |
| 22 | CH2 | 38.7 | 1.57, m; 1.88, m | 38.3 | 1.40, m; 1.50, m | 53 | CH | 135.3 | 5.85, dt, 15.5, 5.7 | 135.6 | 5.82, dt, 15.0, 6.5 |
| 23 | CH2 | 31.3 | 2.40, m | 31.0 | 2.18, m | 54 | CH2 | 34.6 | 1.99, m | 33.2 | 2.10, m |
| 24 | CH | 77.2 | 3.57, m | 77.2 | 3.39, m | 55 | CH2 | 29.9 | 1.30, m | 30.8 | 1.44, m |
| 25 | CH | 72.8 | 3.86, m | 72.5 | 3.71, m | 56–62 | CH2 | 30.1–30.8 | 1.21–1.30, m | 30.0-30.6 | 1.33–1.35, m |
| 26 | CH2 | 41.9 | 1.65, m; 2.16, m | 41.6 | 1.56, 2.02 | 63 | CH2 | 34.6 | 1.97, m | 33.4 | 2.08, m |
| 27 | CH | 71.8 | 4.00, m | 71.4 | 3.90, m | 64 | CH | 140.1 | 5.77, m | 135.8 | 5.81, m |
| 28 | CH2 | 36.9 | 1.67, m | 36.7 | 1.63, m; 1.71, m | 65 | CH2 | 115.0 | 4.91, brd, 11.0; | 114.3 | 4.94, brd, 10.7; |
| 27 | CH | 71.8 | 4.00, m | 71.4 | 3.90, m | 64 | CH | 140.1 | 4.97, brd, 17.0 | 135.8 | 5.01 f |
| 29 | CH2 | 36.4 | 2.12, m; 2.27, m | 36.4 | 2.16, m; 2.24, m | 66 | CH3 | 21.3 | 0.91, d, 7.0 | 20.8 | 0.99, d, 6.9 |
| 30 | C | 138.4 | - | 139.2 | - | 67 | CH3 | 14.2 | 1.05, d, 6.5 | 13.7 | 0.97, d, 7.0 |
| 31 | CH | 126.1 | 5.66, d, 9.0 | 126.0 | 5.52, d, 8.5 | 68 | CH3 | 17.4 | 1.75, s | 17.0 | 1.78, s |
| 32 | CH | 67.5 | 4.76, dd, 9.0, 2.0 | 67.6 | 4.58, dd, 8.5, 1.5 | 69 | CH2 | 113.0 | 5.03, s; 5.17, s | 112.7 | 5.01 f, s; 5.10, s |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutignano, A.; Nuzzo, G.; Sardo, A.; Fontana, A. The Missing Piece in Biosynthesis of Amphidinols: First Evidence of Glycolate as a Starter Unit in New Polyketides from Amphidinium carterae. Mar. Drugs 2017, 15, 157. https://doi.org/10.3390/md15060157
Cutignano A, Nuzzo G, Sardo A, Fontana A. The Missing Piece in Biosynthesis of Amphidinols: First Evidence of Glycolate as a Starter Unit in New Polyketides from Amphidinium carterae. Marine Drugs. 2017; 15(6):157. https://doi.org/10.3390/md15060157
Chicago/Turabian StyleCutignano, Adele, Genoveffa Nuzzo, Angela Sardo, and Angelo Fontana. 2017. "The Missing Piece in Biosynthesis of Amphidinols: First Evidence of Glycolate as a Starter Unit in New Polyketides from Amphidinium carterae" Marine Drugs 15, no. 6: 157. https://doi.org/10.3390/md15060157
APA StyleCutignano, A., Nuzzo, G., Sardo, A., & Fontana, A. (2017). The Missing Piece in Biosynthesis of Amphidinols: First Evidence of Glycolate as a Starter Unit in New Polyketides from Amphidinium carterae. Marine Drugs, 15(6), 157. https://doi.org/10.3390/md15060157