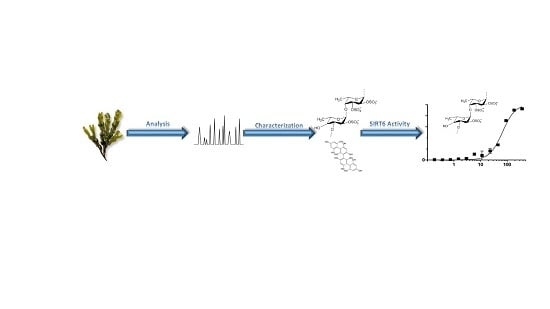
The Identification of a SIRT6 Activator from Brown Algae Fucus distichus
Abstract
:1. Introduction
2. Results
2.1. Screening of Brown Algae
2.2. Separation of F. distichus
2.3. Identification of Fucoidan
2.4. Western Blot Analysis
2.5. Selectivity for SIRT6
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Extraction
4.2.1. Method of Preparation
4.2.2. Species Name
4.3. SIRT6 Deacetylation Assay
4.4. HPLC Analysis
4.5. HPLC Fingerprint
4.6. SemiPREP MS
4.7. H3K9 Western Blot Method
4.8. In Vitro Enzymatic Assays (SIRT1-SIRT3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Gertler, A.A.; Cohen, H.Y. SIRT6, a protein with many faces. Biogerontology 2013, 14, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Puigserver, P. Certainly can’t live without this: SIRT6. Cell Metab. 2006, 3, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The histone deacetylase sirt6 regulates glucose homeostasis via HIF-1 alpha. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Dominy, J.E., Jr.; Lee, Y.; Jedrychowski, M.P.; Chim, H.; Jurczak, M.J.; Camporez, J.P.; Ruan, H.B.; Feldman, J.; Pierce, K.; Mostoslavsky, R.; et al. The deacetylase SIRT6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol. Cell 2012, 48, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, M.; Mao, Z.; Gorbunova, V.; Seluanov, A. Sirt6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell Cycle 2011, 10, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Sociali, G.; Magnone, M.; Ravera, S.; Damonte, P.; Vigliarolo, T.; Von Holtey, M.; Vellone, V.; Millo, E.; Caffa, I.; Cea, M.; et al. Pharmacological sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Khan, S.; Wang, Y.; Charron, G.; He, B.; Sebastian, C.; Du, J.; Kim, R.; Ge, E.; Mostoslavsky, R.; et al. Sirt6 regulates tnf-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 2013, 496, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Qin, C.Y. SIRT6 suppresses hepatocellular carcinoma cell growth via inhibiting the extracellular signalregulated kinase signaling pathway. Mol. Med. Rep. 2014, 9, 882–888. [Google Scholar] [PubMed]
- Feldman, J.L.; Baeza, J.; Denu, J.M. Activation of the protein deacetylase sirt6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J. Biol. Chem. 2013, 288, 31350–31356. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Kokkola, T.; Jarho, E.; Lahtela-Kakkonen, M.; Moaddel, R. N-acylethanolamines bind to sirt6. Chembiochem 2016, 17, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, R.C.; Sabu, A.S.; Chatterji, A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evid. Based Complement. Altern. Med. 2011, 2011, 673083. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jonsdottir, R.; Olafsdottir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective effect of diphlorethohydroxycarmalol against ultraviolet b radiation-induced DNA damage by inducing the nucleotide excision repair system in hacat human keratinocytes. Mar. Drugs 2015, 13, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Falque, E.; Dominguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solidliquid extraction methods on the phenolic content and antioxidant activities of irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Lahtela-Kakkonen, M.; Moaddel, R. Sirtuin 6 (SIRT6) activity assays. Methods Mol. Biol. 2016, 1436, 259–269. [Google Scholar] [PubMed]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Beress, A.; Wassermann, O.; Tahhan, S.; Bruhn, T.; Beress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-hiv compounds (polysaccharides and polyphenols) from the marine alga fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [PubMed]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An unfractionated fucoidan from ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Percival, E.G.V.; Ross, A.G. The isolation and purification of fucoidin from brown seaweeds. J. Chem. Soc. 1950, 717–720. [Google Scholar] [CrossRef]
- Soeda, S.; Kozako, T.; Iwata, K.; Shimeno, H. Oversulfated fucoidan inhibits the basic fibroblast growth factor-induced tube formation by human umbilical vein endothelial cells: Its possible mechanism of action. Biochim. Biophys. Acta-Mol. Cell Res. 2000, 1497, 127–134. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochemical. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Soeda, S.; Shibata, Y.; Shimeno, H. Inhibitory effect of oversulfated fucoidan on tube formation by human vascular endothelial cells. Biol. Pharm. Bull. 1997, 20, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wang, Y.; Zhang, X.; Kim, D.D.; Sadhukhan, S.; Hao, Q.; Lin, H. SIRT7 is activated by DNA and deacetylates histone h3 in the chromatin context. ACS Chem. Biol. 2016, 11, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Buczek-Thomas, J.A.; Hsia, E.; Rich, C.B.; Foster, J.A.; Nugent, M.A. Inhibition of histone acetyltransferase by glycosaminoglycans. J. Cell. Biochem. 2008, 105, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The effect of sulfated (1→3)-alpha-l-fucan from the brown alga saccharina cichorioides miyabe on resveratrol-induced apoptosis in colon carcinoma cells. Mar. Drugs 2013, 11, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer t24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from undaria pinnatifida induces apoptosis in a549 human lung carcinoma cells. Phytother Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Min, E.Y.; Kim, I.H.; Lee, J.; Kim, E.Y.; Choi, Y.H.; Nam, T.J. The effects of fucodian on senescence are controlled by the p16ink4a-prb and p14arf-p53 pathways in hepatocellular carcinoma and hepatic cell lines. Int. J. Oncol. 2014, 45, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tu, B.; Wang, H.; Cao, Z.; Tang, M.; Zhang, C.; Gu, B.; Li, Z.; Wang, L.; Yang, Y.; et al. Tumor suppressor p53 cooperates with sirt6 to regulate gluconeogenesis by promoting foxo1 nuclear exclusion. Proc. Natl. Acad. Sci. USA 2014, 111, 10684–10689. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, M.; He, Z.Z. Low-molecular-weight fucoidan attenuates mitochondrial dysfunction and improves neurological outcome after traumatic brain injury in aged mice: Involvement of sirt3. Cell. Mol. Neurobiol. 2016, 36, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentao, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, P.H.; Leppanen, J.; Rinne, V.M.; Suuronen, T.; Kyrylenko, O.; Kyrylenko, S.; Kuusisto, E.; Tervo, A.J.; Jarvinen, T.; Salminen, A.; et al. N-(3-(4-hydroxyphenyl)-propenoyl)-amino acid tryptamides as sirt2 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2448–2451. [Google Scholar] [CrossRef] [PubMed]
- Tervo, A.J.; Kyrylenko, S.; Niskanen, P.; Salminen, A.; Leppanen, J.; Nyronen, T.H.; Jarvinen, T.; Poso, A. An in silico approach to discovering novel inhibitors of human sirtuin type 2. J. Med. Chem. 2004, 47, 6292–6298. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, P.H.; Suuronen, T.; Wallen, E.A.; Leppanen, J.; Tervonen, J.; Kyrylenko, S.; Salminen, A.; Poso, A.; Jarho, E.M. N(epsilon)-thioacetyl-lysine-containing tri-, tetra-, and pentapeptides as SIRT1 and SIRT2 inhibitors. J. Med. Chem. 2009, 52, 2153–2156. [Google Scholar] [CrossRef] [PubMed]







| Fucoidan (µg/mL) | Fold Increase in Activity ± SD | ||
|---|---|---|---|
| SIRT1 | SIRT2 | SIRT3 | |
| 10 | 0.96 ± 0.04 | 0.87 ± 0.06 | 0.99 ± 0.01 |
| 100 | 0.94 ± 0.02 | 0.82 ± 0.02 | 0.98 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahnasto-Rilla, M.K.; McLoughlin, P.; Kulikowicz, T.; Doyle, M.; Bohr, V.A.; Lahtela-Kakkonen, M.; Ferrucci, L.; Hayes, M.; Moaddel, R. The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Mar. Drugs 2017, 15, 190. https://doi.org/10.3390/md15060190
Rahnasto-Rilla MK, McLoughlin P, Kulikowicz T, Doyle M, Bohr VA, Lahtela-Kakkonen M, Ferrucci L, Hayes M, Moaddel R. The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Marine Drugs. 2017; 15(6):190. https://doi.org/10.3390/md15060190
Chicago/Turabian StyleRahnasto-Rilla, Minna K., Padraig McLoughlin, Tomasz Kulikowicz, Maire Doyle, Vilhelm A. Bohr, Maija Lahtela-Kakkonen, Luigi Ferrucci, Maria Hayes, and Ruin Moaddel. 2017. "The Identification of a SIRT6 Activator from Brown Algae Fucus distichus" Marine Drugs 15, no. 6: 190. https://doi.org/10.3390/md15060190
APA StyleRahnasto-Rilla, M. K., McLoughlin, P., Kulikowicz, T., Doyle, M., Bohr, V. A., Lahtela-Kakkonen, M., Ferrucci, L., Hayes, M., & Moaddel, R. (2017). The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Marine Drugs, 15(6), 190. https://doi.org/10.3390/md15060190