A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geobacillus sp. E263 Infection and Fermentation
2.2. Extraction and Isolation of Bacterial Metabolites
2.3. Cell Proliferation Assay
2.4. Identification of Isolated Compound and the Synthesis of Its Derivatives
2.5. Detection of Apoptosis
2.6. Measurement of Intracellular ROS (Reactive Oxygen Species)
2.7. Statistical Analysis
3. Results
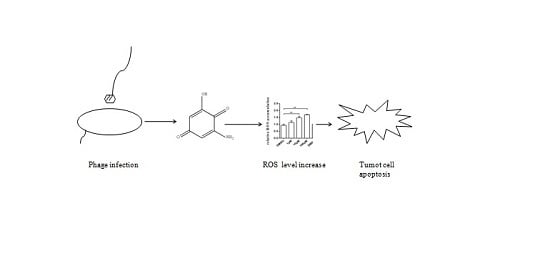
3.1. Novel Anti-Tumor Compound Isolated from Bacteriophage-Challenged Thermophileof Deep-Sea Hydrothermal Vent
3.2. Effects of Derivatives of the Anti-Tumor Compound on the Tumor Cell Proliferation
3.3. Influence of Anti-Tumor Compound and Its Derivatives on Apoptosis of Tumor Cells
3.4. Mechanism of Anti-Tumor Compound-Induced Apoptosis of Tumor Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Farnaes, L.; Coufal, N.G.; Kauffman, C.A.; Rheingold, A.L.; Di Pasquale, A.G.; Jensen, P.R.; Fenical, W. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J. Nat. Prod. 2014, 77, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Friedman, A.J.; Choi, H.; Hogan, J.; McCammon, J.A.; Hook, V.; Gerwick, W.H. The marine cyanobacterial metabolite gallinamide A is a potent and selective inhibitor of human cathepsin L. J. Nat. Prod. 2014, 77, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Das, D.S.; Ray, A.; Richardson, P.G.; Trikha, M.; Anderson, K.C. Effect of combination of proteasome inhibitor marizomib and immunomodulatory agent pomalidomide on synergistic cytotoxicity in multiple myeloma. J. Clin. Oncol. 2014, 32, 8588. [Google Scholar]
- Thornburg, C.C.; Zabriskie, T.M.; McPhail, K.L. Deep-sea hydrothermal vents: Potentialhot spots for natural products discovery? J. Nat. Prod. 2010, 73, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, X. Proteomic analysis of interactions between a deep-sea thermophilic bacteriophage and its host at high temperature. J. Virol. 2010, 84, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, N.Y.; May, A.L.; Middleton, J.L.; Jones, D.R.; Hadden, M.K.; Gooding, J.R.; LeCleir, G.R.; Wilhelm, S.W.; Campagna, S.R.; Buchan, A. Phage infection of an environmentally relevant marine bacterium alters host metabolism and lysate composition. ISME J. 2014, 8, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Xu, C.; Zhang, X. The effect of tryptophol on the bacteriophage infection in high-temperature environment. Appl. Microbiol. Biotechnol. 2015, 99, 8101–8111. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.K.; Letai, A. Control of Mitochondrial Apoptosis by the Bcl-2 Family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tang, Q.; Li, Y.; Hu, B.R.; Ming, Z.Y.; Fu, Q.; Qian, J.Q.; Xiang, J.Z. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol. Sin. 2006, 27, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Mirza, Z.; ASHRAF, G.M.; Kamal, M.A.; Ansari, S.A.; Damanhouri, G.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Sheikh, I.A. New anticancer agents: Recent developments in tumor therapy. Anticancer Res. 2012, 32, 2999–3005. [Google Scholar] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial genome mining for accelerated natural products discovery: Is a renaissance in the making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Öllinger, K.; Kågedal, K. Induction of Apoptosis by Redox-Cycling Quinones. In Phospholipid Metabolism in Apoptosis; Quinn, P.J., Kagan, V.E., Eds.; Springer: Boston, MA, USA, 2002; pp. 151–170. [Google Scholar]
- Doughan, A.K.; Dikalov, S.I. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox Signal. 2007, 9, 1825–1836. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Sun, X.; Jin, M.; Zhang, X. A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells. Mar. Drugs 2017, 15, 200. https://doi.org/10.3390/md15070200
Xu C, Sun X, Jin M, Zhang X. A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells. Marine Drugs. 2017; 15(7):200. https://doi.org/10.3390/md15070200
Chicago/Turabian StyleXu, Chenxi, Xumei Sun, Min Jin, and Xiaobo Zhang. 2017. "A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells" Marine Drugs 15, no. 7: 200. https://doi.org/10.3390/md15070200
APA StyleXu, C., Sun, X., Jin, M., & Zhang, X. (2017). A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells. Marine Drugs, 15(7), 200. https://doi.org/10.3390/md15070200