In Vitro Anticancer and Proapoptotic Activities of Steroidal Glycosides from the Starfish Anthenea aspera
Abstract
:1. Introduction
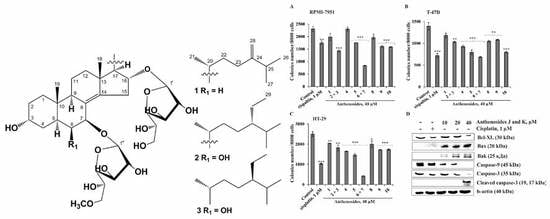
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. Bioactivity Assay
3.5.1. Reagents
3.5.2. Cell Lines and Culture Conditions
3.5.3. Cell Viability Assay
3.5.4. The Soft Agar Colony Formation Assay
3.5.5. Western Blotting
3.5.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C. Global, regional and national cancer incidence, mortality, years of life lost, years lived with disability, and disability adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the sea: Marine compounds as inhibitors of the hallmarks of cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Riccio, R.; Zollo, F. Steroidal oligoglycosides and polyhydroxysteroids from Echinoderms. Fortschr. Chem. Org. Naturst. 1993, 62, 75–308. [Google Scholar] [PubMed]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Iorizzi, M.; De Marino, S.; Zollo, F. Steroidal oligoglycosides from the Asteroidea. Curr. Org. Chem. 2001, 5, 951–973. [Google Scholar] [CrossRef]
- Stonik, V.A.; Ivanchina, N.V.; Kicha, A.A. New polar steroids from starfish. Nat. Prod. Commun. 2008, 3, 1587–1610. [Google Scholar]
- Dong, G.; Xu, T.H.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y.H. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Advances in Natural Products Discovery; Gomes, A.R., Rocha-Santos, T., Duarte, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 191–224. [Google Scholar]
- Ma, N.; Tang, H.F.; Qiu, F.; Lin, H.W.; Tian, X.R.; Zhang, W. A new polyhydroxysteroidal glycoside from the starfish Anthenea chinensis. Chin. Chem. Lett. 2009, 20, 1231–1234. [Google Scholar] [CrossRef]
- Ma, N.; Tang, H.F.; Qiu, F.; Lin, H.W.; Tian, X.R.; Yao, M.N. Polyhydroxysteroidal glycosides from the starfish Anthenea chinensis. J. Nat. Prod. 2010, 73, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kharchenko, S.D.; Kicha, A.A.; Ivanchina, N.V.; Dmitrenok, P.S.; Chingizova, E.A.; Pislyagin, E.A.; Evtushenko, E.V.; Antokhina, T.I.; Minh, C.V.; et al. Anthenosides L‒U, steroidal glycosides with unusual structural features from the starfish Anthenea aspera. J. Nat. Prod. 2016, 79, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ha, D.T.; Ivanchina, N.V.; Malyarenko, T.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Ermakova, S.P.; Malyarenko, O.S.; Hung, N.A.; Thuy, T.T.T.; et al. Six new polyhydroxysteroidal glycosides, anthenosides S1–S6, from the starfish Anthenea sibogae. Chem. Biodivers. 2018, 15, 1700553. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Ivanchina, N.V.; Malyarenko, O.S.; Kalinovsky, A.I.; Dmitrenok, P.S.; Evtushenko, E.V.; Minh, C.V.; Kicha, A.A. Two new steroidal monoglycosides, anthenosides A1 and A2, and revision of the structure of known anthenoside A with unusual monosaccharide residue from the starfish Anthenea aspera. Molecules 2018, 23, 1077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, G.; Tang, H.F.; Zhang, X. Novaeguinoside II inhibits cell proliferation and induces apoptosis of human brain glioblastoma U87MG cells through the mitochondrial pathway. Brain Res. 2011, 1372, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, C.; Li, X.; Zhang, Z.; Yuan, Y.; Ni, Y.; Liu, T.; Deng, S.; Zhao, J.; Wang, Y. Asterosaponin 1 induces endoplasmic reticulum stress-associated apoptosis in A549 human lung cancer cells. Oncol. Rep. 2011, 26, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Shubina, L.K.; Kicha, A.A.; Ivanchina, N.V.; Kwak, J.Y.; Jin, J.O.; Bode, A.M.; Dong, Z.; Stonik, V.A. Proapoptotic and anticarcinogenic activities of leviusculoside G from the starfish Henricia leviuscula and probable molecular mechanism. Nat. Prod. Commun. 2008, 3, 1575–1580. [Google Scholar]
- Kicha, A.A.; Kalinovsky, A.I.; Ivanchina, N.V.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Four new asterosaponins, hippasteriosides A–D, from the Far Eastern starfish Hippasteria kurilensis. Chem. Biodivers. 2011, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Vishchuk, O.S.; Stonik, V.A. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids 2014, 87, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Cariniferosides A-F and other steroidal biglycosides from the starfish Asteropsis carinifera. Steroids 2011, 76, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Rathinam, M.K.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, 51998. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Malyarenko (Vishchuk), O.S.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Kicha, A.A. Four new sulfated polar steroids from the Far Eastern starfish Leptasterias ochotensis: Structures and activities. Mar. Drugs 2015, 13, 4418–4435. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervloessem, H.A.T.; Kiviluoto, S.; Bittremieux, M.; Parys, J.B.; De Smedt, H.; Bultyn, G. A dual role for the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus endoplasmic reticulum. Biochim. Biophys. Acta 2014, 1843, 2240–2252. [Google Scholar] [Green Version]
- Malyarenko, O.S.; Dyshlovoy, S.A.; Kicha, A.A.; Ivanchina, N.V.; Malyarenko, T.V.; Carsten, B.; von Gunhild, A.; Stonik, V.A.; Ermakova, S.P. The Inhibitory Activity of luzonicosides from the starfish Echinaster luzonicus against human melanoma cells. Mar. Drugs 2017, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Ota, E.; Nagashima, Y.; Shiomi, K.; Sakurai, T.; Kojima, C.; Waalkes, M.P.; Himeno, S. Caspase-independent apoptosis induced in rat liver cells by plancitoxin I, the major lethal factor from the crown-of-thorns starfish Acanthaster planci venom. Toxicon 2006, 48, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Hsieh, H.J.; Hwang, D.F. Cytotoxic and apoptotic activities of the plancitoxin I from the venom of crown-of-thorns starfish (Acanthaster planci) on A375.S2 cells. J. Appl. Toxicol. 2015, 35, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Agafonova, I.G.; Stonik, V.A. Steroidal triglycosides, kurilensosides A, D, and C, and other polar steroids from the Far Eastern starfish Hippasteria kurilensis. J. Nat. Prod. 2008, 71, 793–798. [Google Scholar] [CrossRef]



| Position | 1 | Mixture of 2 and 3 | ||||
|---|---|---|---|---|---|---|
| DEPT | δH | δC | DEPT | δH | δC | |
| 1β 1α | CH2 | 1.52 dd (12.3, 5.1) 1.39 m | 32.8 | CH2 | 1.53 m 1.30 m | 34.5 |
| 2 | CH2 | 1.62 m | 29.6 | CH2 | 1.62 m | 29.9 |
| 3 | CH | 3.98 t (2.6) | 67.1 | CH | 4.08 m | 67.5 |
| 4β 4α | CH2 | 1.44 m 1.37 m | 36.3 | CH2 | 1.96 td (13.6, 2.7) 1.37 m | 33.3 |
| 5 | CH | 2.17 m | 33.1 | CH | 2.12 dt (13.6, 2.7) | 38.0 |
| 6 | CH2 | 1.55 dt (14.2, 2.8) 1.23 m | 34.3 | CH | 3.62 t (2.7) | 75.2 |
| 7 | CH | 4.40 t (2.8) | 74.0 | CH | 4.22 d (2.7) | 78.3 |
| 8 | C | 128.9 | C | 127.0 | ||
| 9 | CH | 2.25 m | 46.3 | CH | 2.26 m | 45.9 |
| 10 | C | 38.6 | C | 38.8 | ||
| 11β 11α | CH2 | 1.65 m 1.45 m | 19.8 | CH2 | 1.65 m 1.54 m | 19.5 |
| 12β 12α | CH2 | 1.81 m 1.26 m | 37.2 | CH2 | 1.80 dt (12.5, 3.6) 1.24 m | 37.1 |
| 13 | C | 44.8 | C | 45.0 | ||
| 14 | C | 144.9 | C | 147.3 | ||
| 15β 15α | CH2 | 2.77 ddd (16.8, 8.7, 3.0) 2.56 ddd (16.8, 4.6, 1.8) | 33.7 | CH2 | 2.88 ddd (17.1, 9.1, 3.2) 2.59 ddd (17.1, 5.5, 2.0) | 33.7 |
| 16 | CH | 4.43 td (8.7, 4.6) | 78.2 | CH | 4.49 td (9.1, 5.5) | 77.3 |
| 17 | CH | 1.46 t (4.6) | 62.9 | CH | 1.47 dd (9.1, 3.9) | 63.0 |
| 18 | CH3 | 0.90 s | 20.2 | CH3 | 0.93 s | 20.2 |
| 19 | CH3 | 0.67 s | 11.6 | CH3 | 0.85 s | 15.4 |
| 20 | CH | 1.67 m | 33.3 | CH | 1.60 m | 33.5 33.4 |
| 21 | CH3 | 1.05 d (6.6) | 21.4 | CH3 | 1.05 d (6.9) | 21.7 21.6 |
| 22 | CH2 | 1.81 m 1.43 m | 33.9 | CH2 | 1.65 m 1.26 m | 33.2 |
| 23 | CH2 | 2.23 m 1.95 m | 33.7 | CH2 | 1.47 m 1.09 m | 29.7 29.4 |
| 24 | C | 157.7 | CH | 1.05 m | 47.2 47.0 | |
| 25 | CH | 2.26 m | 35.0 | CH | 1.75 m | 30.2 30.5 |
| 26 | CH3 | 1.04 d (6.7) | 22.5 | CH3 | 0.86 d (6.8) | 20.1 |
| 27 | CH3 | 1.04 d (6.7) | 22.3 | CH3 | 0.85 d (6.8) | 19.3 19.4 |
| 28 | CH2 | 4.76 brs 4.72 brd (1.3) | 107.1 | CH2 | 1.37 m 1.20 m | 24.0 24.2 |
| 29 | CH3 | 0.89 t (7.4) | 12.7 12.4 | |||
| β-d-Galf | ||||||
| 1′ | CH | 4.94 brd (2.4) | 107.6 | 4.96 brd (2.2) | 107.7 | |
| 2′ | CH | 3.95 m | 83.6 | 3.96 dd (4.6, 2.2) | 83.7 | |
| 3′ | CH | 4.03 dd (7.0, 4.8) | 78.3 | 4.04 dd (7.0, 4.6) | 78.4 | |
| 4′ | CH | 3.88 dd (7.0, 2.9) | 84.4 | 3.89 dd (7.0, 3.0) | 84.6 | |
| 5′ | CH | 3.72 m | 72.4 | 3.73 ddd (7.6, 4.5, 3.0) | 72.5 | |
| 6′ | CH2 | 3.61 dd (11.3, 7.5) 3.58 dd (11.3, 4.8) | 65.5 | 3.64 dd (11.2, 7.6) 3.61 dd (11.2, 4.5) | 65.4 | |
| 6-OMe-β-d-Galf | ||||||
| 1′ | CH | 4.99 brd (2.0) | 107.3 | 4.99 brd (1.9) | 108.4 | |
| 2′ | CH | 3.93 dd (3.9, 2.0) | 83.5 | 3.91 dd (3.9, 1.9) | 83.4 | |
| 3′ | CH | 3.95 m | 78.8 | 3.95 dd (6.1, 3.9) | 78.7 | |
| 4′ | CH | 3.90 dd (6.1, 3.7) | 84.8 | 3.87 dd (6.1, 3.6) | 85.0 | |
| 5′ | CH | 3.83 m | 70.8 | 3.84 m | 70.7 | |
| 6′ | CH2 | 3.53 dd (10.1, 4.6) 3.50 dd (10.1, 7.2) | 75.4 | 3.53 d (6.0) | 75.5 | |
| OCH3 | CH3 | 3.38 s | 59.3 | 3.39 s | 59.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyarenko, T.V.; Malyarenko, O.S.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. In Vitro Anticancer and Proapoptotic Activities of Steroidal Glycosides from the Starfish Anthenea aspera. Mar. Drugs 2018, 16, 420. https://doi.org/10.3390/md16110420
Malyarenko TV, Malyarenko OS, Kicha AA, Ivanchina NV, Kalinovsky AI, Dmitrenok PS, Ermakova SP, Stonik VA. In Vitro Anticancer and Proapoptotic Activities of Steroidal Glycosides from the Starfish Anthenea aspera. Marine Drugs. 2018; 16(11):420. https://doi.org/10.3390/md16110420
Chicago/Turabian StyleMalyarenko, Timofey V., Olesya S. Malyarenko, Alla A. Kicha, Natalia V. Ivanchina, Anatoly I. Kalinovsky, Pavel S. Dmitrenok, Svetlana P. Ermakova, and Valentin A. Stonik. 2018. "In Vitro Anticancer and Proapoptotic Activities of Steroidal Glycosides from the Starfish Anthenea aspera" Marine Drugs 16, no. 11: 420. https://doi.org/10.3390/md16110420
APA StyleMalyarenko, T. V., Malyarenko, O. S., Kicha, A. A., Ivanchina, N. V., Kalinovsky, A. I., Dmitrenok, P. S., Ermakova, S. P., & Stonik, V. A. (2018). In Vitro Anticancer and Proapoptotic Activities of Steroidal Glycosides from the Starfish Anthenea aspera. Marine Drugs, 16(11), 420. https://doi.org/10.3390/md16110420