New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation
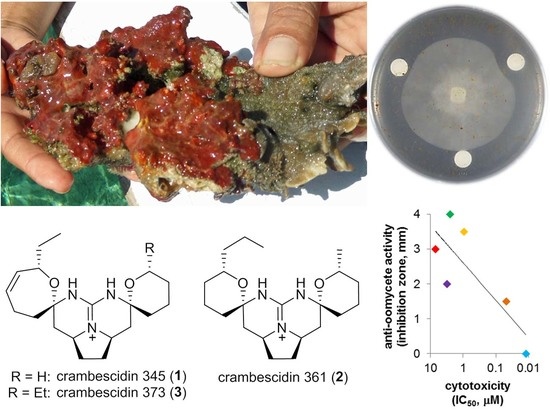
2.2. Biological Activity
3. Materials and Methods
3.1. General Procedures
3.2. Isolation of Bioactive Compounds
3.2.1. Crambescidin 345 (1)
3.2.2. Crambescidin 361 (2)
3.2.3. Crambescidin 373 (3)
3.2.4. Crambescidin 359 (4)
3.2.5. Crambescidin 657 (5)
3.2.6. Crambescidin 800 (6)
3.3. Anti-oomycetete Assay
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Proksch, P. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon 1994, 32, 639–655. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1998, 15, 113–158. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.; Santos, O.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Dewapriya, P. Bioactive compounds from marine sponges and their symbiotic microbes: A potential source of nutraceuticals. Adv. Food Nutr. Res. 2012, 65, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2014, 28, 328–340. [Google Scholar] [CrossRef]
- Huffard, C.L.; Erdmann, M.V.; Gunawan, T.R.P. Geographic Priorities for Marine Biodiversity Conservation in Indonesia; Ministry of Marine Affairs and Fisheries and Marine Protected Areas Governance Program: Jakarta, Indonesia, 2012; pp. 1–6. ISBN 978-602-98450-6-8. [Google Scholar]
- Pawlik, J.R. The chemical ecology of sponges on Caribbean reefs: Natural products shape natural systems. BioScience 2011, 61, 888–898. [Google Scholar] [CrossRef]
- Veron, J.E.N.; Devantier, L.M.; Turak, E.; Green, A.L.; Kininmonth, S.; Stafford-Smith, M.; Peterson, N. Delineating the coral triangle. Galaxea J. Coral Reef Stud. 2009, 11, 91–100. [Google Scholar] [CrossRef]
- Sabdono, A.; Radjasa, O.K. Microbial symbionts in marine sponges: Marine natural product factory. J. Coast. Dev. 2008, 11, 57–61. [Google Scholar]
- Perdicaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive natural substances from marine sponges: New developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1. [Google Scholar] [CrossRef]
- Gomez, P. The genus Clathria from the Gulf of Mexico and Mexican Caribbean, with description and resurrection of Clathria carteri (Poecilosclerida: Microcionidae). Zootaxa 2014, 3790, 51–85. [Google Scholar] [CrossRef] [PubMed]
- Zea, S.; Rodriguez, A.; Martinez, A.M. Taxonomy of Clathria (Thalysias) (Demospongiae: Poecilosclerida: Microcionidae) from the Colombian Caribbean, with description of three new species. Zootaxa 2014, 3835, 401–436. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Miller, M.; Rooney, F. Mirabilin, G: A new alkaloid from a southern Australian marine sponge, Clathria species. J. Nat. Prod. 2001, 64, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Zuleta, I.A.; Vitelli, M.L.; Baggio, R.; Garland, M.T.; Seldes, A.M.; Palermo, J.A. Novel pteridine alkaloids from the sponge Clathria sp. Tetrahedron 2002, 58, 4481–4486. [Google Scholar] [CrossRef]
- Laville, R.; Thomas, O.P.; Berrue, F.; Marquez, D.; Vacelet, J.; Amade, P. Bioactive guanidine alkaloids from two Caribbean marine sponges. J. Nat. Prod. 2009, 72, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Conte, M.; Capon, R.J. Mirabilins revisited: Polyketide alkaloids from a southern Australian marine sponge, Clathria sp. Org. Biomol. Chem. 2010, 8, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Henriksen, N.M.; Skalicky, J.J.; Harper, M.K.; Cheatham, T.E.; Ireland, C.M.; Van Wagoner, R.M. Araiosamines A-D: Tris-bromoindole cyclic guanidine alkaloids from the marine sponge Clathria (Thalysias) araiosa. J. Org. Chem. 2011, 76, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, S.; Ference, C.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. A potent antimicrobial compound isolated from Clathria cervicornis. Bioorg. Med. Chem. 2015, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Katayama, T. Biochemical studies on carotenoids in Porifera. The structure of cathriaxanthin in sea sponge, Clathria frondifera (Bowerbank). Bull. Jpn. Soc. Sci. Fish. 1976, 42, 801–805. [Google Scholar] [CrossRef]
- Dattelbaum, J.D.; Sieg, D.; Minieri, C.M.; Thomson, G.; Hill, M. Plasticity of acquired secondary metabolites in Clathria prolifera (Demospongia: Poecilosclerida): Putative photoprotective role of carotenoids in a temperate intertidal sponge. Open Mar. Biol. J. 2010, 4, 87–95. [Google Scholar] [CrossRef]
- Davis, R.A.; Mangalindan, G.C.; Bojo, Z.P.; Antemano, R.R.; Rodriguez, N.O.; Concepcion, G.P.; Samson, S.C.; de Guzman, D.; Cruz, L.J.; Tasdemir, D.; et al. Microcionamides A and B, bioactive peptides from the Philippine sponge Clathria (Thalysias) abietina. J. Org. Chem. 2004, 69, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; MacLeod, J.K. 5-Thio-d-mannose from the marine sponge Clathria pyramida (Lendenfeld). The first example of a naturally occurring 5-thiosugar. J. Chem. Soc. Chem. Commun. 1987, 1200–1201. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, U.; Schulz, T.C.; McLean, A.B.; Robins, A.J.; West, L.M. Bicyclic C21 terpenoids from the marine sponge Clathria compressa. J. Nat. Prod. 2012, 75, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.-K.; Kim, C.-K.; Ahn, C.-H.; Oh, D.-C.; Oh, K.-B.; Shin, J. Additional sesterterpenes and a nortriterpene saponin from the sponge Clathria gombawuiensis. J. Nat. Prod. 2015, 78, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rudi, A.; Yosief, T.; Loya, S.; Hizi, A.; Schleyer, M.; Kashman, Y. Clathsterol, a novel anti-HIV-1 RT sulfated sterol from the sponge Clathria species. J. Nat. Prod. 2001, 64, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Santalova, E.A.; Makarieva, T.N.; Gorshkova, I.A.; Dmitrenok, A.S.; Krasokhin, V.B.; Stonik, V.A. Sterols from six marine sponges. Biochem. Syst. Ecol. 2004, 32, 153–167. [Google Scholar] [CrossRef]
- Keyzars, R.A.; Northcote, P.T.; Webb, V. Clathriol, a novel polyoxygenated 14β steroid isolated from the New Zealand marine sponge Clathria lissosclera. J. Nat. Prod. 2002, 65, 598–600. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Tavares, R.; Hadju, E.; Van Soest, R.W.M. Novel polycyclic guanidine alkaloids from two marine sponges of the genus Monanchora. J. Nat. Prod. 2000, 63, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Coffey, D.S.; McDonald, A.I.; Overman, L.E.; Rabinowitz, M.H.; Renhowe, P.A. A practical entry to the crambescidin family of guanidine alkaloids. Enantioselective total syntheses of ptilomycalin A, crambescidin 657 and its methyl ester (neofolitispates 2), and crambescidin 800. J. Am. Chem. Soc. 2000, 122, 4893–4903. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Sakai, R.; Rinehart, K.L. Crambescidins: New antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem. 1991, 56, 5712–5715. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Whittaker, N.F.; Bewley, C.A. Crambescidin 826 and dehydrocrambine A: New polycyclic guanidine alkaloids from the marine sponge Monanchora sp. that inhibit HIV-1 fusion. J. Nat. Prod. 2003, 66, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Rodrigues-Stien, A.S.; Petek, S.; Demoy-Schneider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic guanidine alkaloids from a French Polynesian Monanchora n. sp. sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.J. The infra-red spectrum and structure of guanidine. Trans. Faraday Soc. 1959, 55, 524–531. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Lee, H.-S.; Stonik, V.A. Monanchomycalins A and B, unusual guanidine alkaloids from the sponge Monanchora pulchra. Tetrahedron Lett. 2012, 53, 4228–4231. [Google Scholar] [CrossRef]
- Tabakmakher, K.M.; Denisenko, V.A.; Guzii, A.G.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Lee, H.-S.; Makarieva, T.N. Monanchomycalin C, a new pentacyclic guanidine alkaloid from the far-eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2013, 8, 1399–1402. [Google Scholar] [PubMed]
- Cong, H.-J.; Zhang, S.-W.; Shen, Y.; Huang, Y.-J.; Wang, W.-Q.; Leng, Y.; Xuan, L.-J. Guanidine alkaloids from Plumbago zeylanica. J. Nat. Prod. 2013, 76, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Trindade-Silva, A.E.; Santos, M.F.C. The chemistry and biology of organic guanidine derivatives. Nat. Prod. Rep. 2012, 29, 1382–1406. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Frenking, G. Y-conjugated compounds: The equilibrium geometries and electronic structures of guanidine, guanidinium cation, urea, and 1,1-diaminoethylene. J. Am. Chem. Soc. 1993, 115, 2362–2372. [Google Scholar] [CrossRef]
- Feichtinger, K.; Zapf, C.; Sings, H.L.; Goodman, M. Diprotected triflylguanidines: A new class of guanidinylation reagents. J. Org. Chem. 1998, 63, 3804–3805. [Google Scholar] [CrossRef]
- Kashman, Y.; Hirsh, S.; McConnell, O.J.; Ohtani, I.; Kusumi, T.; Kakisawa, H. Ptilomycalin A: A novel polycyclic guanidine alkaloid of marine origin. J. Am. Chem. Soc. 1989, 111, 8925–8926. [Google Scholar] [CrossRef]
- Bensemhoun, J.; Bombarda, I.; Aknin, M.; Vacelet, J.; Gaydou, E.M. Ptilomycalin D, a polycyclic guanidine alkaloid from the marine sponge Monanchora dianchora. J. Nat. Prod. 2007, 70, 2033–2035. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.-E.; Wolfender, J.-L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; Frederich, M.; Bordignon, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Unguiculin A and ptilomycalins E-H, antimalarial guanidine alkaloids from the marine sponge Monanchora unguiculata. J. Nat. Prod. 2017, 80, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Ingrum, A.L.; Carney, J.R.; Rinehart, K.L.; Sakai, R. Polycyclic guanidine-containing compounds from the Mediterranean sponge Crambe crambe: The structure of 13,14,15-isocrambescidin 800 and the absolute stereochemistry of the pentacyclic guanidine moieties of the crambescidins. J. Org. Chem. 1993, 58, 4805–4808. [Google Scholar] [CrossRef]
- Bondu, S.; Genta-Jouve, G.; Leiros, M.; Vale, C.; Guigonis, J.-M.; Botana, L.M.; Thomas, O.P. Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. RSC Adv. 2012, 2, 2828–2835. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Shubina, L.K.; Kuzmich, A.S.; Lee, H.-S.; Stonik, V.A. Monanchocidins B-E: Polycyclic guanidine alkaloids with potent antileukemic activities from the sponge Monanchora pulchra. J. Nat. Prod. 2011, 74, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Stonik, V.A. Monanchocidin: A new apoptosis-inducing polycyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett. 2010, 12, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.D.; Pietraszkiewicz, H.; Overman, L.E.; Valeriote, F.; Cuevas, C. Synthesis and anticancer activity of side chain analogs of the crambescidin alkaloid. Bioorg. Med. Chem. Lett. 2004, 14, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.D.; Overman, L.E. Total synthesis and properties of the crambescidin core zwitterionic acid and crambescidin 359. J. Am. Chem. Soc. 2005, 127, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.E.H.; Nitcheu, J.; Mahmoudi, N.; Ibana, J.A.; Mangalindan, G.C.; Black, G.P.; Howard-Jones, A.G.; Moore, C.G.; Thomas, D.A.; Mazier, D.; et al. Antimalarial activity of crambescidin 800 and synthetic analogues against liver and blood stage of Plasmodium sp. J. Antibiot. 2006, 59, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.G.; Murphy, P.J.; Williams, H.L.; McGown, A.T.; Smith, N.K. Synthetic studies towards ptilomycalin A: Total synthesis of crambescidin 359. Tetrahedron 2007, 63, 11771–11780. [Google Scholar] [CrossRef]
- Van Soest, R.W.M. Marine sponges from Curacao and other Caribbean localities. Part III. Poecilosclerida. Stud. Fauna Curacao Caribb. Isl. 1984, 66, 1–167. [Google Scholar]
- Rinehart, K.L.; Shi, J.-G.; Sun, F. Crambescidin Compounds. US Patent 6028077, 22 February 2000. [Google Scholar]
- Sun, Y.; Tomura, T.; Sato, J.; Iizuka, T.; Fudou, R.; Ojika, M. Isolation and biosynthetic analysis of haliamide, a new PKS-NRPS hybrid metabolite from the marine myxobacterium Haliangium ochraceum. Molecules 2016, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kakuda, T.; Qi, J.; Hirata, M.; Shintani, T.; Yoshioka, Y.; Okamoto, T.; Oba, Y.; Nakamura, H.; Ojika, M. Novel relationship between the antifungal activity and cytotoxicity of marine-derived metabolite xestoquinone and its family. Biosci. Biotechnol. Biochem. 2005, 69, 1749–1752. [Google Scholar] [CrossRef] [PubMed]





| Position | 1 a | 2 b | 3 a |
|---|---|---|---|
| 1 | 0.84, t (7.2) | 0.87, t (6.8) | 0.84, t (7.2) |
| 2a | 1.46, m | 1.38, m | 1.46, m |
| 2b | 1.54, m | 1.54, m | |
| 3 | 4.35, brd (10.8) | 1.41, m | 4.33, brd (10.2) |
| 1.47, m | |||
| 4 | 5.50, dt (10.8, 2.1) | 3.63, brt (12.6) | 5.50, dt (11.2, 2.1) |
| 5a | 5.71, m | 1.29, m | 5.71, m |
| 5b | 1.67, m | ||
| 6a | 2.15, dt (15.3, 7.2) | 1.74, m | 2.15, dt (15.3, 7.2) |
| 6b | 2.42, brt (15.3) | 1.85, m | 2.42, brt (15.3) |
| 7a | 1.97, dd (13.5, 6.0) | 1.74, m | 1.97, dd (13.5, 6.0) |
| 7b | 2.27, t (13.5) | 2.27, t (13.5) | |
| 9a | 1.45, t (12.7) | 1.57, t (12.8) c | 1.45, t (12.9) |
| 9b | 2.59, dd (12.7, 4.8) | 2.19, dd (12.8, 4.2) d | 2.59, dd (12.9, 4.8) |
| 10 | 4.03, m | 4.00, m | 4.05, m |
| 11a | 1.75, m | 1.73, m | 1.75, m |
| 11b | 2.31, m | 2.30, m | 2.32, m |
| 12a | 1.75, m | 1.73, m | 1.75, m |
| 12b | 2.31, m | 2.30, m | 2.32, m |
| 13 | 3.96, m | 4.00, m | 4.03, m |
| 14a | 1.53, t (13.0) | 1.59, t (12.8) c | 1.59, t (13.0) |
| 14b | 2.33, dd (13.0, 4.5) | 2.21, dd (12.8, 4.2) d | 2.21, dd (13.0, 4.8) |
| 16a | 1.77, m | 1.74, m | 1.73, m |
| 16b | 1.77, m | ||
| 17a | 1.80, m | 1.74, m | 1.77, m |
| 17b | 1.85, m | 1.85, m | |
| 18a | 1.61, m | 1.26, m | 1.26, m |
| 18b | 1.70, m | 1.70, m | |
| 19 | 3.69, m | 3.74, m | 3.50, m |
| 20 | 1.11, d (9.0) | 1.42, m | |
| 21 | 0.85, t (7.2) |
| Position | 1 a | 2 b | 3 a |
|---|---|---|---|
| 1 | 10.8, CH3 | 13.8, CH3 | 11.3, CH3 |
| 2 | 30.3, CH2 | 19.4, CH2 | 30.3, CH2 |
| 3 | 72.1, CH | 38.7, CH2 | 72.1, CH |
| 4 | 134.2, CH | 71.1, CH | 134.3, CH |
| 5 | 131.4, CH | 31.7, CH2 | 131.4, CH |
| 6 | 24.4, CH2 | 19.5, CH2c | 24.5, CH2 |
| 7 | 38.5, CH2 | 34.7, CH2d | 38.5, CH2 |
| 8 | 85.1, C | 81.6, C | 85.1, C |
| 9 | 37.9, CH2 | 40.4, CH2 | 37.9, CH2 |
| 10 | 54.9, CH | 53.7, CH e | 54.9, CH |
| 11 | 30.8, CH2 | 30.7, CH2 | 30.8, CH2 |
| 12 | 30.8, CH2 | 30.7, CH2 | 30.8, CH2 |
| 13 | 53.5, CH | 53.4, CHe | 53.5, CH |
| 14 | 39.1, CH2 | 40.4, CH2 | 40.4, CH2 |
| 15 | 81.3, C | 81.6, C | 81.5, C |
| 16 | 35.1, CH2 | 34.6, CH2 d | 34.7, CH2 |
| 17 | 19.5, CH2 | 19.7, CH2 c | 19.5, CH2 |
| 18 | 25.9, CH2 | 33.3, CH2 | 31.3, CH2 |
| 19 | 62.6, CH2 | 68.2, CH | 73.3, CH |
| 20 | 149.4, C | 22,0 CH3 | 30.0, CH2 |
| 21 | 149.0, C | 10.2, CH3 | |
| 22 | 149.4, C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasmiati, K.; Yoshioka, Y.; Okamoto, T.; Ojika, M. New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Mar. Drugs 2018, 16, 84. https://doi.org/10.3390/md16030084
Kasmiati K, Yoshioka Y, Okamoto T, Ojika M. New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Marine Drugs. 2018; 16(3):84. https://doi.org/10.3390/md16030084
Chicago/Turabian StyleKasmiati, Kasmiati, Yukio Yoshioka, Tetsuji Okamoto, and Makoto Ojika. 2018. "New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa" Marine Drugs 16, no. 3: 84. https://doi.org/10.3390/md16030084
APA StyleKasmiati, K., Yoshioka, Y., Okamoto, T., & Ojika, M. (2018). New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Marine Drugs, 16(3), 84. https://doi.org/10.3390/md16030084