Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Transformation of (+)-Prelongilene (3) into Compound 5
3.5. Protein Phosphatase 2A Inhibition Assay
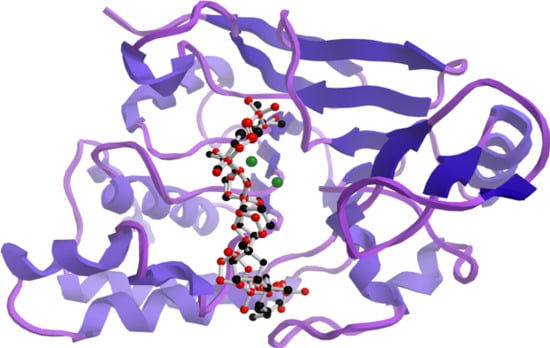
3.6. Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zolnierowicz, S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem. Pharmacol. 2000, 60, 1225–1235. [Google Scholar] [CrossRef]
- Wera, S.; Hemmings, B.A. Serine/threonine protein phosphatases. Biochem. J. 1995, 311, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The Yin and Yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- McCluskey, A.; Sim, A.T.R.; Sakoff, J.A. Serine-Threonine Protein Phosphatase Inhibitors: Development of Potential Therapeutic Strategies. J. Med. Chem. 2002, 45, 1151–1175. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.E.; Golden, T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr. Med. Chem. 2002, 9, 2055–2075. [Google Scholar] [CrossRef] [PubMed]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dilip de Silva, E.; Williams, D.E.; Andersen, R.J.; Klix, H.; Holmes, C.F.B.; Allen, T.M. Motuporin, a Potent Protein Phosphatase Inhibitor Isolated from the Papua New Guinea Sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992, 33, 1561–1564. [Google Scholar] [CrossRef]
- Williams, D.E.; Lapawa, M.; Feng, X.D.; Tarling, T.; Roberge, M.; Andersen, R.J. Spirastrellolide A: Revised structure, progress toward the relative configuration, and inhibition of protein phosphatase 2A. Org. Lett. 2004, 6, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Nordström, L.; Souto, M.L.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Studies on Polyethers Produced by Red Algae. Mar. Drugs 2010, 8, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Villa-Pulgarin, J.A.; Mollinedo, F.; Norte, M.; Daranas, A.H.; Fernández, J.J. Cytotoxic oxasqualenoids from the red alga Laurencia viridis. Eur. J. Med. Chem. 2011, 46, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Cen-Pacheco, F.; Villa-Pulgarin, J.A.; Mollinedo, F.; Norte, M.; Fernández, J.J.; Daranas, A.H. New Polyether Triterpenoids from Laurencia viridis and Their Biological Evaluation. Mar. Drugs 2011, 9, 2220–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, H.; Kishi, E.; Morita, H.; Takeya, K.; Iitaka, Y. A New Squalene-type Triterpene from the Woods of Eurycoma longifolia. Chem. Lett. 1991, 2221–2222. [Google Scholar] [CrossRef]
- Morimoto, Y.; Iwai, T.; Kinoshita, T. Total synthesis and determination of the absolute configuration of (−)-longilene peroxide. Tetrahedron Lett. 2001, 42, 6307–6309. [Google Scholar] [CrossRef]
- Fernández, J.J.; Souto, M.L.; Gil, L.V.; Norte, M. Isolation of naturally occurring dactylomelane metabolites as Laurencia constituents. Tetrahedron 2005, 61, 8910–8915. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olsen, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Velec, H.F.; Gohlke, H.; Klebe, G. DrugScore(CSD)-knowledge-based scoring function derived from small molecule crystal data with superior recognition rate of near-native ligand poses and better affinity prediction. J. Med. Chem. 2005, 48, 6296–6303. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Strack, Z.L.J.; Stock, J.B.; Shi, Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell 2006, 127, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Matsunaga, S.; Takai, A.; Kataiwa, H.; Wakimoto, T.; Fusetani, N.; Isobe, M.; Miki, K. Crystal structure of the complex between calyculin A and the catalytic subunit of protein phosphatase 1. Structure 2002, 10, 715–724. [Google Scholar] [CrossRef]




| (+)-Longilene Peroxide (1) | Longilene (2) | |||
|---|---|---|---|---|
| Position | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 26.9, CH3 | 1.19, s | 29.6, CH3 | 1.29, s |
| 2 | 80.1, C | 70.4, C | ||
| 3 | 137.0, CH | 5.43, d (15.6) | 141.2, CH | 5.62, d (15.6) |
| 4 | 125.8, CH | 5.81, ddd (6.6, 8.5, 15.6) | 122.0, CH | 5.77, ddd (7.0, 7.4, 15.6) |
| 5 | 41.3, CH2 | 1.78, dd (8.5, 13.3) 2.20, dd (6.6, 13.3) | 40.4, CH2 | 1.78, dd (7.0, 13.4) 2.15, dd (7.4, 13.4) |
| 6 | 73.9, C | 74.0, C | ||
| 7 | 85.1, CH | 3.72, m | 84.9, CH | 3.70, dd (6.4, 6.6) |
| 8 | 25.8, CH2 | 1.89, m 2.06, m | 25.6, CH2 | 1.91, m 2.03, m |
| 9 | 29.7, CH2 | 1.49, m 2.06, m | 30.0, CH2 | 1.47, m 2.06, m |
| 10 | 85.8, C | 85.7, C | ||
| 11 | 85.8, CH | 4.09, m | 85.4, CH | 4.11, dd (5.5, 5.6) |
| 12 | 30.1, CH2 | 1.50, m 2.01, m | 30.0, CH2 | 1.49, m 2.01, m |
| 13 | 29.9, CH2 | 1.50, m 2.01, m | 30.0, CH2 | 1.49, m 2.01, m |
| 14 | 85.4, CH | 4.09, m | 85.4, CH | 4.11, dd (5.5, 5.6) |
| 15 | 85.4, C | 85.7, C | ||
| 16 | 29.3, CH2 | 1.46, m 2.03, m | 30.0, CH2 | 1.47, m 2.06, m |
| 17 | 25.2, CH2 | 1.89, m 2.03, m | 25.6, CH2 | 1.91, m 2.03, m |
| 18 | 84.1, CH | 3.72, m | 84.9, CH | 3.70, dd (6.4, 6.6) |
| 19 | 73.8, C | 74.0, C | ||
| 20 | 40.8, CH2 | 1.88, dd (6.8, 13.4) 2.20, dd (7.0, 13.4) | 40.4, CH2 | 1.78, dd (7.0, 13.4) 2.15, dd (7.4, 13.4) |
| 21 | 122.2, CH | 5.75, ddd (6.8, 7.0, 15.6) | 122.2, CH | 5.77, ddd (7.0, 7.4, 15.6) |
| 22 | 141.2, CH | 5.61, d (15.6) | 141.2, CH | 5.62, d (15.6) |
| 23 | 70.0, C | 70.4, C | ||
| 24 | 29.4, CH3 | 1.27, s | 29.6, CH3 | 1.29, s |
| 25 | 24.2, CH3 | 1.37, s | 29.8, CH3 | 1.31, s |
| 26 | 24.3, CH3 | 1.20, s | 24.1, CH3 | 1.24, s |
| 27 | 24.2, CH3 | 1.09, s | 23.7, CH3 | 1.10, s |
| 28 | 23.6, CH3 | 1.07, s | 23.7, CH3 | 1.10, s |
| 29 | 24.3, CH3 | 1.27, s | 24.1, CH3 | 1.24, s |
| 30 | 29.6, CH3 | 1.31, s | 29.8, CH3 | 1.31, s |
| -OOH | 10.57, s | |||
| -OH-6 | 5.24, s | |||
| -OH-19 | 5.03, s | |||
| -OH-23 | 3.29, s | |||
| (+)-Prelongilene (3) | |||||
|---|---|---|---|---|---|
| Position | δC Type | δH (J in Hz) | C | δC Type | δH (J in Hz) |
| 1 | 17.7, CH3 | 1.60, s | 16 | 30.0, CH2 | 1.46, m 2.06, m |
| 2 | 131.1, C | 17 | 25.5, CH2 | 1.93, m 2.14, m | |
| 3 | 124.8, CH | 5.08, t (6.9) | 18 | 85.0, CH | 3.81, dd (4.4, 8.1) |
| 4 | 22.5, CH2 | 1.97, m 2.04, m | 19 | 74.1, C | |
| 5 | 39.0, CH2 | 1.29, m 1.43, m | 20 | 40.8, CH2 | 1.83, m 2.17, m |
| 6 | 72.5, C | 21 | 122.7, CH | 5.74, ddd (6.2, 8.6, 15.2) | |
| 7 | 83.4, CH | 3.73, dd (6.9, 7.6) | 22 | 141.0, CH | 5.62, d (15.2) |
| 8 | 25.4, CH2 | 1.80, m 1.91, m | 23 | 70.3, C | |
| 9 | 30.0, CH2 | 1.46, m 2.04, m | 24 | 29.8, CH3 | 1.31, s |
| 10 | 84.7, C | 25 | 25.7, CH3 | 1.66, s | |
| 11 | 85.0, CH | 4.07, dd (5.4, 10.3) | 26 | 24.9, CH3 | 1.27, s |
| 12 | 29.5, CH2 | 1.50, m 1.99, m | 27 | 23.5, CH3 | 1.08, s |
| 13 | 29.5, CH2 | 1.50, m 1.99, m | 28 | 24.0, CH3 | 1.11, s |
| 14 | 85.7, CH | 4.13, dd (2.1, 5.7) | 29 | 24.1, CH3 | 1.20, s |
| 15 | 85.8, C | 30 | 30.1, CH3 | 1.31, s | |
| OH-6 | 4.94, s | ||||
| OH-19 | 4.39, s | ||||
| OH-23 | 2.57, s | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen-Pacheco, F.; Pérez Manríquez, C.; Luisa Souto, M.; Norte, M.; Fernández, J.J.; Hernández Daranas, A. Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Mar. Drugs 2018, 16, 131. https://doi.org/10.3390/md16040131
Cen-Pacheco F, Pérez Manríquez C, Luisa Souto M, Norte M, Fernández JJ, Hernández Daranas A. Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Marine Drugs. 2018; 16(4):131. https://doi.org/10.3390/md16040131
Chicago/Turabian StyleCen-Pacheco, Francisco, Claudia Pérez Manríquez, María Luisa Souto, Manuel Norte, José Javier Fernández, and Antonio Hernández Daranas. 2018. "Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity" Marine Drugs 16, no. 4: 131. https://doi.org/10.3390/md16040131
APA StyleCen-Pacheco, F., Pérez Manríquez, C., Luisa Souto, M., Norte, M., Fernández, J. J., & Hernández Daranas, A. (2018). Marine Longilenes, Oxasqualenoids with Ser-Thr Protein Phosphatase 2A Inhibition Activity. Marine Drugs, 16(4), 131. https://doi.org/10.3390/md16040131