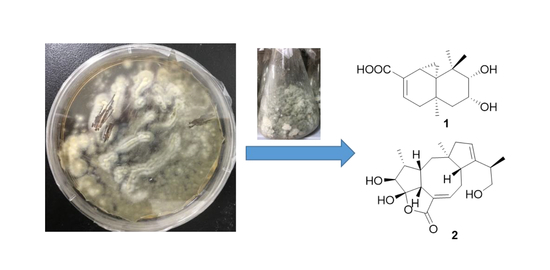
Two New Terpenoids from Talaromyces purpurogenus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Identification of Isolated Terpenoids
2.2. Cytotoxic Activities of Selected Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Isolation
3.4. NMR Calculation
3.5. ECD Calculation
3.6. Cytotoxicity against Cancer Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefrance, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvad, J.C. Phylogeny and nomenclature of the genus talaromyces and taxa accommodated in penicillium subgenus biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centko, R.M.; Williams, D.E.; Patrick, B.O.; Akhtar, Y.; Garcia Chavez, M.A.; Wang, Y.A.; Isman, M.B.; de Silva, E.D.; Andersen, R.J. Dhilirolides E–N, meroterpenoids produced in culture by the fungus Penicillium purpurogenum collected in Sri Lanka: Structure elucidation, stable isotope feeding studies, and insecticidal activity. J. Org. Chem. 2014, 79, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.D.; Williams, D.E.; Jayanetti, D.R.; Centko, R.M.; Patrick, B.O.; Wijesundera, R.L.; Andersen, R.J. Dhilirolides A–D, meroterpenoids produced in culture by the fruit-infecting fungus Penicillium purpurogenum collected in Sri Lanka. Org. Lett. 2011, 13, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, Z.X.; Song, Y.L.; Ren, Y.; Dong, D.; Zheng, J.; Liu, T.; Zhao, Y.F.; Tu, P.F.; Li, J. Anti-neuroinflammatory constituents from the fungus Penicillium purpurogenum MHZ 111. Nat. Prod. Res. 2017, 31, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. Anti-influenza virus polyketides from the acid-tolerant fungus Penicillium purpurogenum JS03-21. J. Nat. Prod. 2011, 74, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, J.; Pan, S.Y.; Gao, J.M.; Tian, J.M. Antifungal, phytotoxic and toxic metabolites produced by Penicillium purpurogenum. Nat. Prod. Res. 2014, 28, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.W.; Cui, C.B.; Li, C.W.; Wu, C.J.; Peng, J.X.; Li, D.H. Rare chromones from a fungal mutant of the marine-derived Penicillium purpurogenum G59. Mar. Drugs 2015, 13, 5219–5236. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Li, C.W.; Cui, C.B. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1815–1838. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, P.; Xu, L.; Wei, X. Penicillitone, a potent in vitro anti-inflammatory and cytotoxic rearranged sterol with an unusual tetracycle core produced by Penicillium purpurogenum. Org. Lett. 2014, 16, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Doi, M.; Tohgo, M.; Minoura, K.; Numata, A. Dankasterone, a new class of cytotoxic steroids produced by a Gymnascella species from a marine sponge. Chem. Commun. 1999, 30, 1321–1322. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in cytostatic constituents of a sponge-derived gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sun, Y.L.; Liu, K.S.; Zhang, X.Y.; Qian, P.Y.; Wang, Y.F.; Qi, S.H. Indole alkaloids from marine-derived fungus Aspergillus sydowii SCSIO 00305. J. Antibiot. 2012, 65, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.L.; Fang, Y.C.; Zhu, T.J.; Gu, Q.Q.; Zhu, W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, J.; Bi, L.; Zhao, M.; Wang, C.; Baudy-Floc’h, M.; Ju, J.; Peng, S. Toward breast cancer resistance protein (BCRP) inhibitors: Design, synthesis of a series of new simplified fumitremorgin C analogues. Tetrahedron 2007, 63, 5510–5528. [Google Scholar] [CrossRef]
- Matsuo, A.; Nakayama, N.; Nakayama, M. Enantiomeric type sesquiterpenoids of the liverwort Marchantia polymorpha. Phytochemistry 1985, 24, 777–781. [Google Scholar] [CrossRef]
- Norin, T.; Jakobsen, H.J.; Larsen, E.H.; Forsén, S.; Meisingseth, E. The chemistry of the natural order Cupressales. 49. The configuration of thujopsene. Acta Chem. Scand. 1963, 17, 738–748. [Google Scholar] [CrossRef]
- Górecki, M.; Jabłońska, E.; Kruszewska, A.; Suszczyńska, A.; Urbańczyklipkowska, Z.; Gerards, M.; Morzycki, J.W.; Szczepek, W.J.; Frelek, J. Practical method for the absolute configuration assignment of tert/tert 1,2-diols using their complexes with Mo2(OAc)4. J. Org. Chem. 2007, 72, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Li, D.Y.; Li, Y.C.; Hua, H.M.; Ma, E.L.; Li, Z.L. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain–many compounds strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, H.; Tanaka, K.; Fukushi, E.; Matsuo, K.; Nehira, T.; Hashimoto, M. Roussoellols A and B, tetracyclic fusicoccanes from Roussoella hysterioides. J. Nat. Prod. 2013, 76, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- White, K.N.; Amagata, T.; Oliver, A.G.; Tenney, K.; Wenzel, P.J.; Crews, P. Structure revision of spiroleucettadine, a sponge alkaloid with a bicyclic core meager in H-atoms. J. Org. Chem. 2009, 73, 8719–8722. [Google Scholar] [CrossRef] [PubMed]
- Rychnovsky, S.D. Predicting NMR spectra by computational methods: Structure revision of hexacyclinol. Org. Lett. 2006, 8, 2895–2898. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhou, J.; Liu, J.; Huang, J.; Zhang, H.; Liu, R.; Yao, G. Acetylcholinesterase inhibitory alkaloids from the whole plants of Zephyranthes carinata. J. Nat. Prod. 2017, 80, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Bao, L.; Yang, X.L.; Liu, D.L.; Guo, H.; Dai, H.Q.; Song, F.H.; Zhang, L.X.; Guo, L.D.; Li, S.J. Ophiobolins P-T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia 2013, 90, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Itoh, T.; Kinoshita, M.; Nakai, Y.; Kurotaki, M.; Kobayashi, M. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron 2004, 60, 6015–6019. [Google Scholar] [CrossRef]
- Liu, H.B.; Edrada-Ebel, R.A.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S.; Müller, W.E.G.; Wray, V.; Lin, W.H.; Proksch, P. Ophiobolin sesterterpenoids and pyrrolidine alkaloids from the sponge-derived fungus Aspergillus ustus. Helv. Chim. Acta 2011, 94, 623–631. [Google Scholar] [CrossRef]
- Zhu, T.; Lu, Z.; Fan, J.; Wang, L.; Zhu, G.; Wang, Y.; Li, X.; Hong, K.; Piyachaturawat, P.; Chairoungdua, A. Ophiobolins from the mangrove fungus Aspergillus ustus. J. Nat. Prod. 2018, 81, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Vainio, M.J.; Johnson, M.S. Generating conformer ensembles using a multiobjective genetic algorithm. J. Chem. Inf. Model. 2007, 47, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Puranen, J.S.; Vainio, M.J.; Johnson, M.S. Accurate conformation-dependent molecular electrostatic potentials for high-throughput in silico drug discovery. J. Comput. Chem. 2010, 31, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Qiao, Y.; Xu, Q.; Hu, Z.; Li, X.N.; Xiang, M.; Liu, J.; Huang, J.; Zhu, H.; Wang, J.; Luo, Z.; et al. Diterpenoids of the cassane type from Caesalpinia decapetala. J. Nat. Prod. 2016, 79, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Liu, J.; Zhou, J.; Sun, B.; Aisa, H.A.; Yao, G. Amaryllidaceae alkaloids with new framework types from Zephyranthes candida as potent acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2017, 127, 771–780. [Google Scholar] [CrossRef] [PubMed]





| Position | 1 | 2 | 2a | 2b | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δC (calcd.) | δC (cor) | δC (calcd.) | δC (cor) | |
| 1 | - | 35.7 | 1.60, d, 13.0 1.40, dd, 14.6, 13.0 | 37.6 | 39.2 | 35.9 | 36.2 | 33.7 |
| 2 | 0.85, dd, 9.1,5.0 | 11.3 | 2.70, m | 41.2 | 45.7 | 42.1 | 38.6 | 36.0 |
| 0.76, d, 5.1 | ||||||||
| 3 | 2.11, dd, 9.0, 5.1 | 17.9 | 2.45, m | 40.8 | 49.6 | 45.9 | 58.7 | 55.1 |
| 4 | - | 134.3 | 3.81, d, 4.4 | 80.0 | 86.0 | 80.7 | 85.4 | 80.6 |
| 5 | 6.56, d, 4.6 | 133.7 | - | 114.1 | 118.4 | 111.7 | 115.2 | 109.0 |
| 6 | 1.88, dd, 18.3, 2.6 | 42.8 | 3.42, br d, 9.4 | 52.8 | 57.9 | 53.8 | 55.4 | 52.0 |
| 1.78, dd, 18.3, 7.0 | ||||||||
| 7 | - | 31.9 | - | 130.5 | 133.5 | 126.2 | 133.7 | 126.7 |
| 8 | 1.57, dd, 14.4, 3.1 | 41.5 | 7.04, m | 141.6 | 156.4 | 148.1 | 148.6 | 140.9 |
| 1.50, dd, 14.4, 3.7 | ||||||||
| 9 | 4.03, ddd, 3.8, 3.7, 3.1 | 72.8 | 2.65, overlap 2.36, m | 28.6 | 31.4 | 28.5 | 31.0 | 28.7 |
| 10 | 3.23, d, 3.8 | 78.6 | 3.19, dd, 13.6, 2.8 | 47.9 | 51.9 | 48.1 | 50.0 | 46.8 |
| 11 | - | 39.9 | - | 46.9 | 53.2 | 49.3 | 51.6 | 48.3 |
| 12 | - | 171.2 | 2.28, dd, 15.1, 4.2 1.76, dd, 15.1, 2.6 | 45.5 | 46.6 | 43.0 | 48.2 | 45.9 |
| 13 | 1.44, s | 30.6 | 5.31, br s | 122.1 | 128.4 | 121.3 | 127.8 | 121.0 |
| 14 | 0.78, s | 25.9 | - | 148.4 | 159.3 | 150.9 | 159.3 | 151.1 |
| 15 | 1.31, s | 23.5 | 2.22, ddq, 7.9, 5.3, 7.4 | 36.7 | 41.6 | 38.2 | 40.8 | 38.1 |
| 16 | 0.93, d, 7.4 | 11.0 | 15.0 | 12.8 | 17.0 | 15.4 | ||
| 17 | - | 173.0 | 178.6 | 169.4 | 184.9 | 175.5 | ||
| 18 | 0.84, s | 24.9 | 25.0 | 22.4 | 25.0 | 23.0 | ||
| 19 | 1.04, d, 6.8 | 17.7 | 17.7 | 15.4 | 17.5 | 15.8 | ||
| 20 | 3.66, dd, 10.6, 5.3 3.37, dd, 10.6, 7.9 | 67.0 | 68.9 | 64.4 | 69.6 | 65.5 | ||
| 1 | 2 | 3 | Adriamycin | |
|---|---|---|---|---|
| SW480 | >40 | 23.6 | 14.2 | 1.2 |
| HL-60 | 12.6 | 10.9 | 7.9 | 0.05 |
| A549 | 35.7 | 25.8 | 21.3 | 0.10 |
| MCF-7 | >40 | 6.5 | 23.8 | 0.80 |
| SMMC-7721 | >40 | >40 | >40 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wan, X.; Liu, J.; Wang, J.; Zhu, H.; Chen, C.; Zhang, Y. Two New Terpenoids from Talaromyces purpurogenus. Mar. Drugs 2018, 16, 150. https://doi.org/10.3390/md16050150
Wang W, Wan X, Liu J, Wang J, Zhu H, Chen C, Zhang Y. Two New Terpenoids from Talaromyces purpurogenus. Marine Drugs. 2018; 16(5):150. https://doi.org/10.3390/md16050150
Chicago/Turabian StyleWang, Wenjing, Xiao Wan, Junjun Liu, Jianping Wang, Hucheng Zhu, Chunmei Chen, and Yonghui Zhang. 2018. "Two New Terpenoids from Talaromyces purpurogenus" Marine Drugs 16, no. 5: 150. https://doi.org/10.3390/md16050150
APA StyleWang, W., Wan, X., Liu, J., Wang, J., Zhu, H., Chen, C., & Zhang, Y. (2018). Two New Terpenoids from Talaromyces purpurogenus. Marine Drugs, 16(5), 150. https://doi.org/10.3390/md16050150