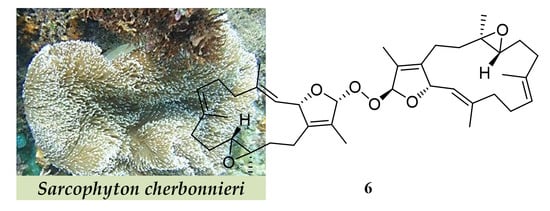
New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.3.1. Cherbonnolide A (1)
3.3.2. Cherbonnolide B (2)
3.3.3. Cherbonnolide C (3)
3.3.4. Cherbonnolide D (4)
3.3.5. Cherbonnolide E (5)
3.3.6. Bischerbolide Peroxide (6)
3.3.7. Reduction of Cherbonolides B and E (2 and 5)
3.3.8. Preparation of (S)- and (R)- MTPA Esters of 1 and 3
3.4. In Vitro Anti-Inflammatory Testing
3.4.1. Human Neutrophils
3.4.2. Superoxide Anion Generation
3.4.3. Elastase Release
3.4.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farag, M.A.; Fekry, M.I.; Al-Hammady, M.A.; Khalil, M.N.; El-Seedi, H.R.; Meyer, A.; Porzel, A.; Westphal, H.; Wessjohann, L.A. Cytotoxic effects of Sarcophyton sp. soft corals-Is there a correlation to their NMR fingerprints? Mar. Drugs 2017, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Pare, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Pare, P.W.; Hegazy, M.E.F. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; ElSohly, M.A.; Hassanean, H.A.; Hassanean, H.A.; Ahmed, S.A. Cytotoxic cembranoids from the Red Sea soft coral, Sarcophyton auritum. Tetrahedron Lett. 2014, 55, 3984–3988. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lu, Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Bioactive cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Su, J.H.; Lu, Y.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Hashimoto, K.; Yokogawa, Y.; Okamura, H.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Cytotoxic biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2009, 72, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Tseng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hsu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha Atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Yang, Y.C.; Wang, S.K.; Duh, C.Y. Numerosol A-D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, H.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; You, W.J.; Lin, C.C.; El-Shazly, M.; Liao, Z.J.; Su, J.H. Anti-Inflammatory cembranoids from the soft coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Tseng, Y.J.; Chen, B.W.; Hwang, T.L.; Chen, H.Y.; Dai, C.F.; Sheu, J.H. Tortuosenes A and B, new diterpenoid metabolites from the Formosan soft coral Sarcophyton tortuosum. Org. Lett. 2014, 16, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wu, C.Y.; Huang, C.Y.; Wang, H.C.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Cubitanoids and cembranoids from the soft coral Sinularia nanolobata. Mar. Drugs 2016, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Huang, C.Y.; Dai, C.F.; Wen, Z.H.; Sheu, J.H. A novel symmetric sulfur-containing biscembranoid from the formosan soft coral Sinularia flexibilis. Tetrahedron Lett 2010, 51, 764–766. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Kurtan, T.; Mandi, A.; Yan, X.H.; Zhang, W.; Guo, Y.W. Biscembranoids formed from an alpha, beta-unsaturated gamma-lactone ring as a dienophile: Structure revision and establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J. Org. Chem. 2013, 78, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, T.; Igari, M.; Ishitsuka, M.O.; Ichikawa, A.; Itezono, Y.; Nakayama, N.; Kakisawa, H. A Novel chlorinated biscembranoid from the marine soft coral Sarcophyton glaucum. J. Org. Chem. 1990, 55, 6286–6289. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Ahmed, A.F.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Sinulochmodins A-C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005, 7, 3813–3816. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pattenden, G. Biomimetic syntheses of ineleganolide and sinulochmodin C from 5-episinuleptolide via sequences of transannular Michael reactions. Tetrahedron 2011, 67, 10045–10052. [Google Scholar] [CrossRef]
- Kusumi, T.; Yamada, K.; Ishitsuka, M.O.; Fujita, Y.; Kakisawa, H. New cembranoids from the Okinawan soft coral Sinularia mayi. Chem. Lett. 1990, 19, 1315–1318. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Kuramoto, J.; Nakayama, M.; Hase, T. Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral Lobophytum denticulatum. Tetrahedron Lett. 1985, 26, 4487–4490. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Pare, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1992, 9, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Chen, W.T.; Li, X.W.; Wang, H.Y.; Guo, Y.W. New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, H.O.; Berger, S.; Braun, S. Carbon-13 NMR Spectroscopy; John Wiley & Sons: Chichester, UK, 1988. [Google Scholar]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and alpha-methoxy-alpha-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Duh, C.Y.; Chia, M.C.; Wang, S.K.; Chen, H.J.; El-Gamal, A.A.; Dai, C.F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Corminboeuf, O.; Overman, L.E.; Pennington, L.D. Total synthesis of the reputed structure of alcyonin and reassignment of its structure. Org. Lett. 2003, 5, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Chen, B.W.; Dai, C.F.; Sung, P.J.; Wu, Y.C.; Sheu, J.H. Sarcophyton F and G new dihydrofuranocembranoids from a Donsha atoll soft coral Sarcophyton sp. Bull. Chem. Soc. Jpn. 2012, 85, 920–922. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Heaton, A.; Konig, G.; Bruck, M.A.; Cramer, R.E.; Klein, D.M.; Scheuer, P.J. The structure of four isometric dihydrofuran-containing cembranoid diterpenes from several species of soft corals. J. Nat. Prod. 1987, 50, 650–659. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C. Studies of Australian soft corals. XLV. Epoxidation reaction of cembranoid diterpenes: Stereochemical outcomes. Heterocycles 1989, 28, 669–672. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirase, T. Marine terpenes and terpenoids. XI. Structures of new dihydrofuranocembranoids isolated from a Sarcophyton sp. soft coral of Okinawa. Chem. Pharm. Bull. 1990, 38, 2442–2445. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free. Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Su, Y.C.; Chang, H.L.; Leu, Y.L.; Chung, P.J.; Kuo, L.M.; Chang, Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Minami, H.; Hayashi, E.; Kodama, M.; Kawazu, K.; Fukuyama, Y. Neovibsanin C, a macrocyclic peroxide-containing neovibsane-type diterpene from Viburnum awabuki. Tetrahedron Lett. 1999, 40, 6261–6265. [Google Scholar] [CrossRef]
- Kamchonwongpaisan, S.; Nilanonta, C.; Tarnchompoo, B.; Thebtaranonth, C.; Thebtaranonth, Y.; Yuthavong, Y.; Kongsaeree, P.; Clardy, J. An antimalarial peroxide from Amomum krervanh Pierre. Tetrahedron Lett. 1995, 36, 1821–1824. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an antibiotic from Plakortis halichondrioides. J. Org. Chem. 1978, 34, 3454–3457. [Google Scholar] [CrossRef]
- Wells, R.J. A novel peroxyketal from a sponge. Tetrahedron Lett. 1976, 17, 2637–2638. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.B.; Hu, L. Natural endoperoxides as drug lead compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free. Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]










| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) c | δC d, type | δH, m (J in Hz) c | δC d, type | |
| 1 | 160.7, C | 160.6, C | 160.6, C | 159.8, C | ||||
| 2 | 5.44, dd (10.0, 1.6) | 77.8, CH | 5.43, dd (10.0, 1.6) | 77.8, CH | 4.91, dd (10.0, 1.6) | 78.4, CH | 4.98, d (10.4) | 77.8, CH |
| 3 | 4.90, d (10.0) | 122.2, CH | 4.91, d (10.0) | 122.7, CH | 4.55, d (10.0) | 123.9, CH | 4.45, d (10.4) | 123.5, CH |
| 4 | 141.6, C | 140.8, C | 140.2, C | 139.4, C | ||||
| 5α | 2.20, m | 47.9, CH2 | 2.18, dd (12.4, 10.8) | 42.6, CH2 | 2.42, dd (12.0, 3.2) | 49.1, CH2 | 1.98, m | 49.3, CH2 |
| 5β | 2.76, dd (12.8, 5.2) | 2.87, dd (12.4, 4.4) | 189, m | 2.25, dd (12.8, 3.6) | ||||
| 6 | 4.70, ddd (10.4, 10.4, 5.2) | 65.2, CH | 4.97, ddd (10.8, 9.2, 4.4) | 78.3, CH | 3.84, dd (9.2, 9.2) | 69.6, CH | 4.21, ddd (11.2, 9.2, 3.6) | 64.8, CH |
| 7 | 5.20, d (10.4) | 128.1, CH | 5.05, d (9.2) | 123.1, CH | 5.09, d (9.2) | 131.6, CH | 4.84, d (9.2) | 131.2, CH |
| 8 | 139.8, C | 144.2, C | 138.4, C | 139.4, C | ||||
| 9α | 2.03, m | 36.8, CH2 | 2.07, m | 36.9, CH2 | 2.21, ddd (13.6, 13.6, 2.4) | 28.2, CH2 | 1.58, m | 28.5, CH2 |
| 9β | 2.38, m | 2.42, m | 1.63, m | 2.30, dd (13.2, 4.8) | ||||
| 10α | 1.29, m | 23.5, CH2 | 1.35, m | 23.6, CH2 | 1.16, m | 23.9, CH2 | 1.60, m | 22.7, CH2 |
| 10β | 2.51, m | 2.17, m | 1.84, m | 1.28, m | ||||
| 11 | 2.42, dd (10.8, 2.8) | 61.4, CH | 2.43, m | 61.4, CH | 2.24, dd (10.4, 2.4) | 58.9, CH | 1.98, m | 62.6, CH |
| 12 | 60.8, C | 60.8, C | 59.7, CH | 60.9, C | ||||
| 13α | 2.03, m | 36.9, CH2 | 2.02, m | 37.0, CH2 | 1.49, m | 35.5, CH2 | 1.61, m | 37.1, CH2 |
| 13β | 1.06, m | 1.07, m | 0.98, m | 0.65, m | ||||
| 14α | 2.49, m | 23.7, CH2 | 2.52, m | 23.7, CH2 | 1.58, m | 22.2, CH2 | 2.08, m | 23.1, CH2 |
| 14β | 2.01, m | 2.03, m | 1.58, m | 1.65, m | ||||
| 15 | 123.8, C | 123.8, C | 123.8, C | 123.7, C | ||||
| 16 | 174.4, C | 174.4, C | 173.9, C | 174.3, C | ||||
| 17 | 1.86, s | 8.8, CH3 | 1.86, s | 8.7, CH3 | 1.64, s | 8.8, CH3 | 1.63, s | 8.8, CH3 |
| 18 | 1.70, s | 15.9, CH3 | 1.72, s | 15.9, CH3 | 1.31, s | 18.1, CH3 | 1.28, s | 16.9, CH3 |
| 19 | 1.86, s | 14.9, CH3 | 1.89, s | 15.3, CH3 | 1.45, s | 22.2, CH3 | 1.32, s | 21.8, CH3 |
| 20 | 1.33, s | 15.8, CH3 | 1.33, s | 15.8, CH3 | 1.00, s | 17.1, CH3 | 0.99, s | 16.4, CH3 |
| 6-OOH | 7.99, br s | |||||||
| Position | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|
| δH, m (J in Hz) a | δC b, type | δH, m (J in Hz) c | δC d, type | δH, m (J in Hz) c | δC d, type | ||
| 1 | 160.4, C | 141.4, C | 1′ | 141.5, C | |||
| 2 | 4.95, d (10.0) | 78.4, CH | 5.28, d (10.0) | 82.7, CH | 2′ | 5.50, d (10.0) | 81.9, CH |
| 3 | 4.42, d (10.0) | 124.6, CH | 5.06, d (10.0) | 126.4, CH | 3′ | 4.92, d (10.0) | 125.1, CH |
| 4 | 139.2, C | 140.2, C | 4′ | 141.0, C | |||
| 5α | 1.95, m | 44.6, CH2 | 2.21, m | 38.5, CH2 | 5′α | 2.21, m | 38.8, CH2 |
| 5β | 2.47, br d (11.0) | 2.32, m | 5′β | 2.31, m | |||
| 6 | 4.58, ddd (11.0, 9.5, 2.5) | 78.9, CH | 24.2, CH2 | 6′ | 24.2, CH2 | ||
| 6α | 2.07, m | 6′α | 2.07, m | ||||
| 6β | 2.42, m | 6′β | 2.42, m | ||||
| 7 | 4.78, d (9.5) | 126.6, CH | 4.98, dd (9.2, 9.2) | 125.6, CH | 7′ | 4.95, dd (9.2, 9.2) | 125.5, CH |
| 8 | 143.8, C | 133.1, C | 8′ | 133.3, C | |||
| 9α | 1.65, m | 29.8, CH2 | 1.96, m | 36.6, CH2 | 9′α | 1.96, m | 36.6, CH2 |
| 9β | 2.52, dd (14.0, 4.5) | 2.27, m | 9′β | 2.27, m | |||
| 10α | 1.28, m | 23.4, CH2 | 1.22, m | 23.6, CH2 | 10′α | 1.22, m | 23.7, CH2 |
| 10β | 1.62, m | 2.04, m | 10′β | 2.04, m | |||
| 11 | 1.97, m | 63.3, CH | 2.51, m | 62.1, CH | 11′ | 2.51, m | 62.2, CH |
| 12 | 61.5, C | 61.2, CH | 12′ | 61.3, C | |||
| 13α | 1.59, m | 37.6, CH2 | 1.83, m | 37.3, CH2 | 13′α | 1.83, m | 37.4, CH2 |
| 13β | 0.64, m | 0.95, m | 13′β | 0.95, m | |||
| 14α | 2.07, m | 23.7, CH2 | 2.33, m | 22.6, CH2 | 14′α | 2.33, m | 22.7, CH2 |
| 14β | 1.61, m | 1.81, m | 14′β | 1.81, m | |||
| 15 | 124.3, C | 124.9, C | 15′ | 124.9, C | |||
| 16 | 174.3, C | 6.13, br s | 114.3, C | 16′ | 6.17, d (3.6) | 114.4, CH | |
| 17 | 1.63, s | 9.4, CH3 | 1.72, s | 10.2, CH3 | 17′ | 1.73, s | 10.2, CH3 |
| 18 | 1.29, s | 17.3, CH3 | 1.58, s | 14.6, CH3 | 18′ | 1.59, s | 14.6, CH3 |
| 19 | 1.34, s | 22.5, CH3 | 1.65, s | 14.7, CH3 | 19′ | 1.65, s | 14.7, CH3 |
| 20 | 0.98, s | 16.9, CH3 | 1.27, s | 15.7, CH3 | 20′ | 1.27, s | 15.7, CH3 |
| 6-OOH | 7.25, br s | ||||||
| Position | 6 | 8 a | 9 a |
|---|---|---|---|
| H-11 | δH 2.51 (H-11, H-11′) | δH 2.50 | δH 2.75 |
| C-11 | δC 62.1 (C-11) | δC 62.3 | δC 61.2 |
| δC 62.2 (C-11′) | |||
| C-12 | δC 61.2 (C-12) | δC 61.4 | δC 60.7 |
| δC 61.3 (C-12′) | |||
| C-13 | δC 37.3 (C-13) | δC 37.4 | δC 35.4 |
| δC 37.4 (C-13′) | |||
| C-14 | δC 22.6 (C-14) | δC 22.5 | δC 20.4 |
| δC 22.7 (C-14′) | |||
| H3-18 | δH 1.58 (H3-18) | δH 1.58 | δH 1.70 |
| δH 1.59 (H3-18′) | |||
| H3-20 | δH 1.27 (H3-20, H3-20′) | δH 1.28 | δH 1.18 |
| C-20 | δC 15.7 (C-20, C-20′) | δC 15.7 | δC 17.7 |
| Compounds | Superoxide Anion | Elastase Release | |
|---|---|---|---|
| IC50 (μM) a | Inh b % | Inh b % | |
| 1 | >30 | 32.1 ± 4.3 ** | 37.6 ± 5.0 ** |
| 2 | >30 | 4.0 ± 6.7 | 23.5 ± 6.6 * |
| 3 | >30 | 44.5 ± 4.6 *** | 35.6 ± 6.2 ** |
| 4 | >30 | 6.4 ± 4.2 | 27.6 ± 6.4 ** |
| 5 | >30 | 2.6 ± 6.2 | 30.5 ± 4.6 ** |
| 6 | 26.2 ± 1.0 | 64.6 ± 0.8 *** | 42.4 ± 5.1 ** |
| 7 | >30 | 3.5 ± 5.3 | 20.7 ± 4.1 ** |
| Idelalisib | 0.07 ± 0.01 | 102.8 ± 2.2 *** | 99.6 ± 4.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. https://doi.org/10.3390/md16080276
Peng C-C, Huang C-Y, Ahmed AF, Hwang T-L, Dai C-F, Sheu J-H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Marine Drugs. 2018; 16(8):276. https://doi.org/10.3390/md16080276
Chicago/Turabian StylePeng, Chia-Chi, Chiung-Yao Huang, Atallah F. Ahmed, Tsong-Long Hwang, Chang-Feng Dai, and Jyh-Horng Sheu. 2018. "New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri" Marine Drugs 16, no. 8: 276. https://doi.org/10.3390/md16080276
APA StylePeng, C. -C., Huang, C. -Y., Ahmed, A. F., Hwang, T. -L., Dai, C. -F., & Sheu, J. -H. (2018). New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Marine Drugs, 16(8), 276. https://doi.org/10.3390/md16080276