Aspergixanthones I–K, New Anti-Vibrio Prenylxanthones from the Marine-Derived Fungus Aspergillus sp. ZA-01
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation of the Fungal Material
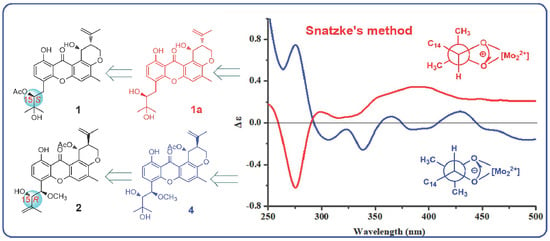
3.3. Preparation of the Methanolysis Derivative (1a) of 1
3.4. Snatzke’s Method
3.5. Anti-Vibrio Activity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masters, K.S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Lesch, B.; Bräse, S. A short, atom-economical entry to tetrahydroxanthenones. Angew. Chem. Int. Ed. 2004, 43, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Liu, H.; Meng, L.H.; Wang, B.G. Two new diphenylketones and a new xanthone from Talaromyces islandicus EN-501, an endophytic fungus derived from the marine red alga Laurencia okamurai. Mar. Drugs 2016, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T. Identification and bioactivity of compounds from the fungus Penicillium sp. CYE-87 isolated from a marine tunicate. Mar. Drugs 2015, 13, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Anja, K.; Stefan, K.; Clarissa, G.; Karin, K.; Martin, N.; Armin, M.; Heinz, H.F.; Iuliana, A.; Gerhard, R.; Jörg, F.; et al. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2007, 70, 353–360. [Google Scholar] [CrossRef]
- Pontius, A.; Krick, A.; Mesry, R.; Kehraus, S.; Foegen, S.E.; Müller, M.; Klimo, K.; Gerhäuser, C.; König, G.M. Monodictyochromes A and B, dimeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2008, 71, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.W.; Yu, G.H.; Kurtán, T.; Mándi, A.; Peng, J.; Mo, X.M.; Liu, M.; Li, H.; Sun, X.H.; Li, J.; et al. Versixanthones A–F, cytotoxic xanthone-chromanone dimers from the marine-derived fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fang, L.Z.; Liu, F.L.; Pang, X.J.; Qin, H.L.; Zhao, T.; Xu, L.L.; Yang, D.F.; Yang, X.L. New prenylxanthones, polyketide hemiterpenoid pigments from the endophytic fungus Emericella sp. XL029 and their anti-agricultural pathogenic fungal and antibacterial activities. RSC Adv. 2017, 7, 31115–31122. [Google Scholar] [CrossRef]
- Zhu, A.; Yang, M.Y.; Zhang, Y.H.; Shao, L.L.; Wang, C.Y.; Hu, L.D.; Cao, F.; Zhu, H.J. Absolute configurations of 14,15-hydroxylated prenylxanthones from a marine-derived Aspergillus sp. fungus by chiroptical methods. Sci. Rep. 2018, 8, 10621. [Google Scholar] [CrossRef] [PubMed]
- Fredimoses, M.; Zhou, X.F.; Lin, X.P.; Tian, X.P.; Ai, W.; Wan, J.F.; Liao, S.R.; Liu, J.; Yang, B.; Yang, X.W.; et al. New prenylxanthones from the deep-sea derived fungus Emericella sp. SCSIO 05240. Mar. Drugs 2014, 1, 3190–3202. [Google Scholar] [CrossRef] [PubMed]
- Chexal, K.K.; Fouweather, C.; Holker, J.S.E.; Simpson, T.J.; Young, K. The biosynthesis of fungal metabolites. Ш. Structure of shamixanthone and tajixanthone, metabolites of Aspergillus variecolor. J. Chem. Soc. Perkin. Trans. 1 1974, 5, 1584–1593. [Google Scholar] [CrossRef]
- Moosophon, P.; Kanokmedhakul, S.K.; Soytong, K. Prenylxanthones and a bicyclo[3.3.1]nona-2,6-diene derivative from the fungus Emericella rugulosa. J. Nat. Prod. 2009, 72, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, S.S.; Zhu, A.; Cao, F.; Zhu, H.J. Bioactive azaphilone derivatives from the fungus Talaromyces aculeatus. J. Nat. Prod. 2017, 80, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- González, A.M.; Rivera, C.J.; Sosa, P.A.; Figueroa, M.; Rodríguez-Sotres, R.; Mata, R. Development of the fluorescent biosensor h Calmodulin (h CaM) L39C-monobromobimane (mBBr)/V91C-mBBr, a novel tool for discovering new calmodulin inhibitors and detecting calcium. J. Med. Chem. 2011, 54, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Gonźalez, M.C.; Rodŕıguez-Sotres, R.; Sosa-Peinado, A.; Gonźalez-Andrade, M.; Cerda-Garćıa-Rojas, C.M.; Mata, R. Calmodulin inhibitors from the fungus Emericella sp. Bioorg. Med. Chem. 2009, 17, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Cao, F.; Shao, C.L.; Chen, M.; Liu, H.J.; Zheng, C.J.; Wang, C.Y. Subergorgiaols A–L, 9,10-secosteroids from the South China Sea gorgonian Subergorgia rubra. Steroids 2015, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Parks, M.; Pinnell, L.J.; Tallman, J.J.; Turner, J.W. Draft genome sequence of a Vibrio harveyi strain associated with vibriosis in Pacific white shrimp (Litopenaeus vannamei). Genome Announc. 2017, 5, e01662-16. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Höfle, M.; Pruzzo, C. Effects of global warming on Vibrio ecology. Microbiol. Spectr. 2015, 3, VE-0004-2014. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 | 2 | 3 |
|---|---|---|---|
| 2 | 6.71, d (8.4) | 6.80, d (8.4) | 6.85, d (8.4) |
| 3 | 7.41, d (8.4) | 7.60, d (8.4) | 7.65, d (8.4) |
| 5 | 7.29, s | 7.27, s | 7.24, s |
| 14 | 3.33, dd (14.4, 2.4) | 4.82, d (8.4) | 4.83, d (8.4) |
| 2.91, dd (14.4, 10.8) | |||
| 15 | 5.15, dd (10.8, 2.4) | 4.19, d (8.4) | 4.19, d (8.4) |
| 17 | 1.34, s | 4.65, brs | 4.64, brs |
| 4.62, brs | 4.60, brs | ||
| 18 | 1.38, s | 1.76, s | 1.77, s |
| 19 | 4.46, brd (10.8) | 4.56, brd (11.4) | 4.43, dd (10.8, 3.0) |
| 4.35, dd (12.0, 10.8) | 4.32, dd (11.4, 3.0) | 4.35, dd (10.8, 3.0) | |
| 20 | 2.55, d (12.0) | 2.72, brs | 2.72, d (3.0) |
| 22 | 5.06, s | 4.81, s | 4.81, s |
| 4.78, s | 4.76, s | 4.59, s | |
| 23 | 1.86, s | 1.89, s | 1.85, s |
| 24 | 2.38, s | 2.36, s | 2.37, s |
| 25 | 5.50, brs | 6.90, brs | 5.43, brs |
| 1-OH | 12.63, brs | 13.06, brs | 12.83, brs |
| 14-OCH3 | - | 3.28, s | 3.30, s |
| 15-OAc | 1.99, s | - | - |
| 25-OH | 4.51, brs | - | 4.96, d (4.2) |
| 25-OAc | - | 2.10, s | - |
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 161.1, C | 162.0, C | 161.8, C |
| 2 | 109.5, CH | 110.7, CH | 110.7, CH |
| 3 | 137.9, CH | 134.7, CH | 135.1, CH |
| 4 | 115.1, C | 115.4, C | 115.8, C |
| 5 | 119.5, CH | 120.4, CH | 119.1, CH |
| 6 | 139.0, C | 138.0, C | 139.0, C |
| 7 | 149.6, C | 150.4, C | 149.9, C |
| 8 | 121.8, C | 115.0, C | 121.4, C |
| 9 | 109.2, C | 109.0, C | 108.8, C |
| 10 | 153.3, C | 153.5, C | 153.7, C |
| 11 | 151.8, C | 151.8, C | 152.0, C |
| 12 | 116.9, C | 116.4, C | 116.9, C |
| 13 | 184.5, C | 183.4, C | 184.5, C |
| 14 | 29.7, CH2 | 78.7, CH | 78.8, CH |
| 15 | 78.6, CH | 80.0, CH | 80.0, CH |
| 16 | 72.5, C | 141.7, C | 142.5, C |
| 17 | 26.9, CH3 | 114.8, CH2 | 114.8, CH2 |
| 18 | 25.3, CH3 | 18.2, CH3 | 18.2, CH3 |
| 19 | 64.1, CH2 | 63.9, CH2 | 64.8, CH2 |
| 20 | 44.1, CH | 42.6, CH | 45.1, CH |
| 21 | 142.3, C | 142.5, C | 142.7, C |
| 22 | 111.7, CH2 | 112.9, CH2 | 112.4, CH2 |
| 23 | 22.5, CH3 | 22.6, CH3 | 22.7, CH3 |
| 24 | 17.4, CH3 | 17.5, CH3 | 17.7, CH3 |
| 25 | 61.0, CH | 65.7, CH | 63.3, CH |
| 14-OCH3 | - | 57.2, CH3 | 57.2, CH3 |
| 15-OAc | 170.4, C | - | - |
| 20.7, CH3 | |||
| 25-OAc | - | 170.2, C | - |
| 21.4, CH3 |
| Strains | Compounds [MIC (μM)] | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Ciprofloxacin | |
| V. parahemolyticus | 1.56 | 6.25 | 3.12 | 25.0 | 12.5 | 6.25 | 25.0 | 0.078 |
| V. anguillarum | 1.56 | 25.0 | 25.0 | 25.0 | 25.0 | 6.25 | 6.25 | 0.312 |
| V. alginolyticus | 3.12 | 25.0 | 12.5 | 25.0 | 12.5 | 12.5 | 25.0 | 0.625 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, A.; Zhang, X.-W.; Zhang, M.; Li, W.; Ma, Z.-Y.; Zhu, H.-J.; Cao, F. Aspergixanthones I–K, New Anti-Vibrio Prenylxanthones from the Marine-Derived Fungus Aspergillus sp. ZA-01. Mar. Drugs 2018, 16, 312. https://doi.org/10.3390/md16090312
Zhu A, Zhang X-W, Zhang M, Li W, Ma Z-Y, Zhu H-J, Cao F. Aspergixanthones I–K, New Anti-Vibrio Prenylxanthones from the Marine-Derived Fungus Aspergillus sp. ZA-01. Marine Drugs. 2018; 16(9):312. https://doi.org/10.3390/md16090312
Chicago/Turabian StyleZhu, Ao, Xing-Wang Zhang, Miao Zhang, Wan Li, Zheng-Yue Ma, Hua-Jie Zhu, and Fei Cao. 2018. "Aspergixanthones I–K, New Anti-Vibrio Prenylxanthones from the Marine-Derived Fungus Aspergillus sp. ZA-01" Marine Drugs 16, no. 9: 312. https://doi.org/10.3390/md16090312
APA StyleZhu, A., Zhang, X. -W., Zhang, M., Li, W., Ma, Z. -Y., Zhu, H. -J., & Cao, F. (2018). Aspergixanthones I–K, New Anti-Vibrio Prenylxanthones from the Marine-Derived Fungus Aspergillus sp. ZA-01. Marine Drugs, 16(9), 312. https://doi.org/10.3390/md16090312