Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Characterization of COS
2.2. NO Production
2.3. Cell Viability
2.4. COS Induced the Production of Cytokines in Macrophages
2.5. COS Enhanced Cytokine Gene and Protein Expression
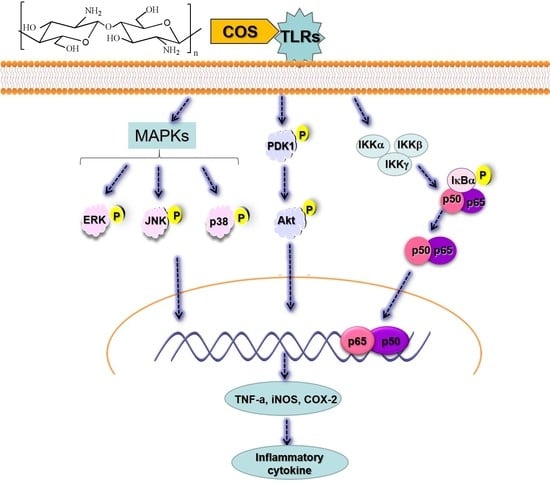
2.6. COS Interferes with the NF-κB, MAPK and PI3K/Akt Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of α- and β-Chitooligosaccharide
4.3. Characterization of Chitosan and Chitooligosaccharide
4.4. Cell Culture
4.5. Cell Proliferation Assay
4.6. NO and Cytokines Quantitation
4.7. The mRNA Expression Levels of Cytokines Determined by Reverse Transcription-Polymerase Chain Reaction
4.8. Immunoblotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diskin, C.; Palsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.W.; Sohng, J.K.; Pandey, R.P.; Park, Y.I. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-kappaB signaling pathway. Int. Immunopharmacol. 2016, 31, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, R. Michael Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, M.-C.; Wang, D.-M.; Hsieh, H.-J. Fabrication of a novel porous PGA-chitosan hybrid matrix for tissue engineering. Biomaterials 2003, 24, 1047–1057. [Google Scholar] [CrossRef]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Xing, R.; Liu, Y.; Li, K.; Yu, H.; Liu, S.; Yang, Y.; Chen, X.; Li, P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohydr. Polym. 2017, 157, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Peng, Y.; Han, B.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.X.; Chen, H.X.; Zhang, J.; Zhang, X.D.; Liang, Y.X. Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. Int. J. Biol. Macromol. 2013, 62, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, Y.; Zhu, Q.; Xiao, J.; Xia, W. Effects of chitosan pentamer and chitosan hexamerin vivoandin vitroon gene expression and secretion of cytokines. Food Agric. Immunol. 2009, 20, 269–280. [Google Scholar] [CrossRef]
- Xiong, Q.; Hao, H.; He, L.; Jing, Y.; Xu, T.; Chen, J.; Zhang, H.; Hu, T.; Zhang, Q.; Yang, X.; et al. Anti-inflammatory and anti-angiogenic activities of a purified polysaccharide from flesh of Cipangopaludina Chinensis. Carbohydr. Polym. 2017, 176, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Fang, L.; Zhao, X.; Zhou, A.; Xie, J. A galactomannoglucan derived from Agaricus brasiliensis: Purification, characterization and macrophage activation via MAPK and IkappaB/NFkappaB pathways. Food Chem. 2018, 239, 603–611. [Google Scholar] [CrossRef]
- Wu, N.; Wen, Z.S.; Xiang, X.W.; Huang, Y.N.; Gao, Y.; Qu, Y.L. Immunostimulative Activity of Low Molecular Weight Chitosans in RAW264.7 Macrophages. Mar. Drugs 2015, 13, 6210–6225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, N.; Shinozawa, Y.; Ishibiki, K.; Osahiko, A. Clinical analysis of multiple organ failure in burned patients. Burns Incl. Therm. Injury 1987, 13, 103–109. [Google Scholar] [CrossRef]
- Von Asmuth, E.J.U.; Maessen, J.G.; Van Der Linden, C.J.; Buurma, W.A. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand. J. Immunol. 1990, 32, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.H.T.; Hendriks, T.; Verhofstad, A.J.A.; Kullberg, B.-J.; Goris, R.J. Improved survival of TNF-deficient mice during the zymosan-induced multiple organ dysfunction syndrome. Shock 2002, 17, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wen, Z.S.; Huang, Y.J.; Xia, M.S.; Xiang, X.W.; Qu, Y.L. Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-kappaB and AP-1 Signaling Pathways in RAW264.7 Macrophages. Mar. Drugs 2016, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, C.; Chen, X.; Wang, J.; Tian, J. Effects of five chitosan oligosaccharides on nuclear factor-kappa B signaling pathway. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2012, 27, 276–279. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm 2014, 2014, 352–371. [Google Scholar] [CrossRef]
- Ma, P.; Liu, H.-T.; Wei, P.; Xu, Q.-S.; Bai, X.-F.; Du, Y.-G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Kim, H.M.; Hong, S.H.; Yoo, S.J.; Baek, K.S.; Jeon, Y.J.; Choung, S.Y. Differential effects of chitooligosaccharides on serum cytokine levels in aged subjects. J. Med. Food 2006, 9, 427–430. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Varum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Wu, G.J.; Tsai, G.J. Chitooligosaccharides in combination with interferon-γ increase nitric oxide production via nuclear factor-κB activation in murine RAW264.7 macrophages. Food Chem. Toxicol. 2007, 45, 250–258. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, L.; Yu, Z.; Feng, J.; Yu, Q. Role of mannose receptor in oligochitosan-mediated stimulation of macrophage function. Int. Immunopharmacol. 2005, 5, 1533–1542. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effects of sulfated chitosans on RAW 264.7 mouse macrophages via the activation of PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2018, 108, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-S.; Hong, Y.D.; Kim, Y.; Sung, N.Y.; Yang, S.; Lee, K.M.; Park, J.Y.; Park, J.S.; Rho, H.S.; Shin, S.S.; et al. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J. Ginseng Res. 2015, 39, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Mar. Drugs 2019, 17, 36. https://doi.org/10.3390/md17010036
Yang Y, Xing R, Liu S, Qin Y, Li K, Yu H, Li P. Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Marine Drugs. 2019; 17(1):36. https://doi.org/10.3390/md17010036
Chicago/Turabian StyleYang, Yue, Ronge Xing, Song Liu, Yukun Qin, Kecheng Li, Huahua Yu, and Pengcheng Li. 2019. "Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways" Marine Drugs 17, no. 1: 36. https://doi.org/10.3390/md17010036
APA StyleYang, Y., Xing, R., Liu, S., Qin, Y., Li, K., Yu, H., & Li, P. (2019). Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Marine Drugs, 17(1), 36. https://doi.org/10.3390/md17010036