Biotechnological Applications of Scyphomedusae
Abstract
:1. Introduction
2. Organic Content of Scyphomedusae
3. Proteins
4. Fatty Acids
5. Bioactive Compounds from Crude Venom
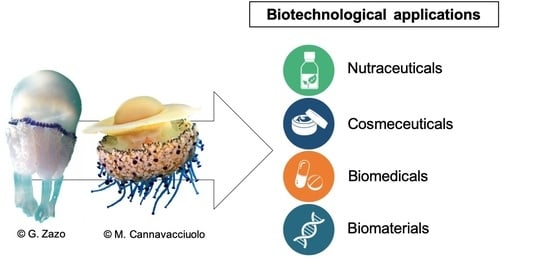
6. Biotechnological Applications of Scyphomedusae: State of the Art and Perspectives
6.1. Nutraceuticals
6.2. Cosmeceuticals
6.3. Biomedical Applications
6.3.1. Collagen
6.3.2. Crude Venom
6.4. Biomaterials
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Querellou, J.; Borresen, T.; Boyen, C.; Dobson, A.; Hofle, M.; Ianora, A.; Jaspars, M.; Kijjoam, A.; Olafsen, J.; Rigos, G.; et al. Marine Biotechnology: A New Vision and Strategy for Europe; European Science Foundation: Ostend, Belgium, 2010; pp. 1–96. [Google Scholar]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.M.; Gelcich, S.; Robinson, K.L.; Duarte, C.M.; Brotz, L.; Purcell, J.E.; Madin, L.P.; Mianzán, H.; Sutherland, K.R.; Uye, S.-I.; et al. Linking human well-being and jellyfish: Ecosystem services, impacts, and societal responses. Front. Ecol. Environ. 2014, 12, 515–523. [Google Scholar] [CrossRef]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiol. 2012, 690, 3–20. [Google Scholar] [CrossRef]
- Brotz, L.; Pauly, D. Jellyfish populations in the Mediterranean Sea. Acta Adriat. 2012, 53, 213–232. [Google Scholar]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef]
- Condon, R.H.; Graham, W.M.; Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Haddock, S.H.D.; Sutherland, K.R.; Robinson, K.L.; Dawson, M.N.; Decker, M.B.; et al. Questioning the rise of gelatinous zooplankton in the world’s oceans. BioScience 2012, 62, 160–169. [Google Scholar] [CrossRef]
- Roux, J.-P.; Van Der Lingen, C.D.; Gibbons, M.J.; Moroff, N.E.; Shannon, L.J.; Smith, A.D.; Cury, P.M. Jellyfication of Marine Ecosystems as a Likely Consequence of Overfishing Small Pelagic Fishes: Lessons from the Benguela. Bull. Mar. Sci. 2013, 89, 249–284. [Google Scholar] [CrossRef] [Green Version]
- Purcell, J.E.; Benovic, A. Potential links of jellyfish to eutrophication and fisheries. In Ecosystems at the Land-Sea Margin: Drainage Basin to Coastal Seas. In Ecosystems at the Land-Sea Margin: Drainage Basin to Coastal Sea; Malone, T., Malej, A., Eds.; American Geophysical Union: Washington, DC, USA, 1999; Volume 55, pp. 241–263. [Google Scholar]
- Purcell, J.E. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Boil. Assoc. UK 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Lucas, C.H.; Pitt, K.A.; Purcell, J.E.; Lebrato, M.; Condon, R.H. What’s in a jellyfish? Proximate and elemental composition and biometric relationships for use in biogeochemical studies. Ecology 2011, 92, 1704. [Google Scholar] [CrossRef]
- Kogovšek, T.; Tinta, T.; Klun, K.; Malej, A. Jellyfish biochemical composition: Importance of standardised sample processing. Mar. Ecol. Prog. Ser. 2014, 510, 275–288. [Google Scholar] [CrossRef]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates: Porifera and cnidaria. Prog. Lipid Res. 1979, 18, 1–30. [Google Scholar] [CrossRef]
- Hooper, S.; Ackman, R. Presence of trans-6-hexadecenoic acid in the White Jellyfish Aurelia aurita Lamarck and in a Caribbean Gorgonian. Lipids 1973, 8, 95. [Google Scholar] [CrossRef]
- Holland, D.L.; Davenport, J.; East, J. The fatty acid composition of the leatherback turtle Dermochelys coriacea and its jellyfish prey. J. Mar. Boil. Assoc. UK 1990, 70, 761–770. [Google Scholar] [CrossRef]
- Abdullah, A.; Nurjanah, N.; Taufik, H.; Dimas, U.A. Fatty acid profile of Jellyfish (Aurelia aurita) as a source raw material of aquatic result rich beneft. Int. J. Chem. Biol. Sci. 2015, 1, 12–16. [Google Scholar]
- Schneider, G. Chemische Zusammensetzung und Biomasseparameter der Ohrenqualle Aurelia aurita. Helgol. Meeresunters. 1988, 42, 319–327. [Google Scholar] [CrossRef]
- Lucas, C.; Lucas, C. Biochemical composition of Aurelia aurita in relation to age and sexual maturity. J. Exp. Mar. Boil. Ecol. 1994, 183, 179–192. [Google Scholar] [CrossRef]
- Kariotoglou, D.M.; Mastronicolis, S.K. Sphingophosphonolipids, phospholipids, and fatty acids from Aegean jellyfish Aurelia aurita. Lipids 2001, 36, 1255–1264. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sato, H.; Yoshie-Stark, Y.; Ogushi, M.; Tanaka, Y. Differences in the biochemical compositions of two dietary jellyfish species and their effects on the growth and survival of Ibacus novemdentatus phyllosomas. Aquac. Nutr. 2016, 22, 25–33. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [Green Version]
- Doyle, T.K.; Houghton, J.D.R.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-consumption. J. Exp. Mar. Biol. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Bailey, T.; Youngbluth, M.; Owen, G. Chemical composition and metabolic rates of gelatinous zooplankton from midwater and benthic boundary layer environments off Cape Hatteras, North Carolina, USA. Mar. Ecol. Prog. Ser. 1995, 122, 121–134. [Google Scholar] [CrossRef]
- Sipos, J.C.; Ackman, R.G. Jellyfish (Cyanea capillata) Lipids: Fatty Acid Composition. J. Fish. Res. Board Can. 1968, 25, 1561–1569. [Google Scholar] [CrossRef]
- Malej, A. Rates of Metabolism of Jellyfish as Related to Body Weight, Chemical Composition and Temperature, Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Athens, Greece, 1991; UNEP, Ed.; UNEP: Athens, Greece, 1991; pp. 253–259. [Google Scholar]
- Malej, A.; Faganeli, J.; Pezdič, J. Stable isotope and biochemical fractionation in the marine pelagic food chain: The jellyfish Pelagia noctiluca and net zooplankton. Mar. Boil. 1993, 116, 565–570. [Google Scholar] [CrossRef]
- Nakhel, I.C.; Mastronicolis, S.K.; Miniadis-Meimaroglou, S. Phospho- and phosphonolipids of the aegean pelagic scyphomedusa Pelagia noctiluca. Biochim. Biophys. Acta BBA Lipids Lipid Metabol. 1988, 958, 300–307. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Mooney, B.D.; Nichols, P.D. Lipids of gelatinous antarctic zooplankton: Cnidaria and Ctenophora. Lipids 2000, 35, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Morais, Z.B.; Pintão, A.M.; Costa, I.M.; Calejo, M.T.; Bandarra, N.M.; Abreu, P. Composition and In Vitro Antioxidant Effects of Jellyfish Catostylus tagi from Sado Estuary (SW Portugal). J. Aquat. Food Prod. Technol. 2009, 18, 90–107. [Google Scholar] [CrossRef]
- Carli, A.; Pane, L.; Valente, T.; Cotta, S. Lipid and Protein Content of Jellyfish from the Ligurian Sea. First Results, Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Athens, Greece, 1991; UNEP, Ed.; UNEP: Athens, Greece, 1991; pp. 236–240. [Google Scholar]
- Gubareva, A.E.; Pustovalova, L.M.; Ryzhkov, Y.D. The features of the chemical composition of medusae in the sea of Azov. Izvestiya Severo Kavkazskogo Nauchnogo Tsentra Vysshei Shkoly Estestvennye Nauki 1983, 0, 22–25. [Google Scholar]
- Prieto, L.; Enrique-Navarro, A.; Volsi, R.L.; Ortega, M.J. The Large Jellyfish Rhizostoma luteum as Sustainable a Resource for Antioxidant Properties, Nutraceutical Value and Biomedical Applications. Mar. Drugs 2018, 16, 396. [Google Scholar] [CrossRef]
- Huang, Y.-W. Cannonball jellyfish (Stomolophus meleagris) as a food resource. J. Food Sci. 1988, 53, 341–343. [Google Scholar] [CrossRef]
- Reinhardt, S.B.; Van Vleet, E.S.; Vleet, E.S. Lipid composition of twenty-two species of Antarctic midwater zooplankton and fish. Mar. Boil. 1986, 91, 149–159. [Google Scholar] [CrossRef]
- Clarke, A.; Holmes, L.J.; Gore, D.J. Proximate and elemental composition of gelatinous zooplankton from the Southern Ocean. J. Exp. Mar. Boil. Ecol. 1992, 155, 55–68. [Google Scholar] [CrossRef]
- Müller, W.E.G. The Origin of Metazoan complexity: Porifera as integrated animals 1. Integr. Comp. Biol. 2003, 43, 3–10. [Google Scholar] [CrossRef]
- Szpak, P. Fish bone chemistry and ultrastructure: Implications for taphonomy and stable isotope analysis. J. Archaeol. Sci. 2011, 38, 3358–3372. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Lin, Z. In vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Boil. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Khan, S.B.; Qian, Z.-J.; Ryu, B.; Kim, S.-K. Isolation and biochemical characterization of collagens from seaweed pipefish, Syngnathus schlegeli. Biotechnol. Bioprocess Eng. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Senaratne, L.; Park, P.-J.; Kim, S.-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Thuy, L.T.M.; Okazaki, E.; Osako, K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, I.; E Sikorski, Z.; Niecikowska, C. Parameters affecting the isolation of collagen from squid (Illex argentinus) skins. Food Chem. 1999, 66, 153–157. [Google Scholar] [CrossRef]
- Shanmugam, V. Extraction, structural and physical characterization of type I collagen from the outer skin of Sepiella inermis (Orbigny, 1848). Afr. J. Biotechnol. 2012, 11, 14326–14337. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Eriksson, A.; Burcharth, J.; Rosenberg, J. Animal derived products may conflict with religious patients’ beliefs. BMC Med. Eth. 2013, 14, 48. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [Green Version]
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Calejo, M.T.; Morais, Z.B.; Fernandes, A.I. Isolation and Biochemical Characterisation of a Novel Collagen from Catostylus tagi. J. Biomater. Sci. Polym. Ed. 2009, 20, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for uUse in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Miura, S.; Kimura, S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. J. Boil. Chem. 1985, 260, 15352–15356. [Google Scholar]
- Iverson, S.J. Tracing aquatic food webs using fatty acids: From qualitative indicators to quantitative determination. In Lipids in Aquatic Ecosystems; Arts, M.T., Kainz, M., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 2009; pp. 281–308. [Google Scholar]
- Russo, G.L. Dietary n−6 and n−3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Chapter 13—Health benefits of n-3 polyunsaturated fatty acid: Eicosapentaenoic acid and docosahexaenoic acid. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, pp. 211–222. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Cripps, G.C.; Atkinson, A. Fatty acid composition as an indicator of carnivory in Antarctic krill, Euphausia superba. Can. J. Fish. Aquat. Sci. 2000, 57, 31–37. [Google Scholar] [CrossRef]
- Trathan, P.N.; Hill, S.L. The Importance of Krill Predation in the Southern Ocean. In Biology and Ecology of Antarctic Krill; Siegel, V., Ed.; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 321–350. [Google Scholar]
- Boero, F. Review of Jellyfish Blooms in the Mediterranean and Black Sea; FAO: Rome, Italy, 2013; Volume 92, p. 53. [Google Scholar]
- Nicolas, J.-P.; Lin, Y.; Lambeau, G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H. Localization of Structural Elements of Bee Venom Phospholipase A2Involved in N-type Receptor Binding and Neurotoxicity. J. Boil. Chem. 1997, 272, 7173–7181. [Google Scholar] [CrossRef]
- Ackman, R.; Hooper, S.; Sipos, J. Distribution of trans-6-hexadecenoic and other fatty acids in tissues and organs of the atlantic leatherback turtle Dermochelys coriacea coriacea L. Int. J. Biochem. 1972, 3, 171–179. [Google Scholar] [CrossRef]
- Crowell, S.; Oates, S. Metamorphosis and reproduction by transverse fission in an Edwardsiid anemone. In Developmental and Cellular Biology of Coelenterates; Tardent, P., Tardent, R., Eds.; Elsevier: Amsterdam, The Nerderlands; North Holland Biomedical Press: New York, NY, USA, 1980; pp. 139–142. [Google Scholar]
- Fukuda, Y.; Naganuma, T. Potential dietary effects on the fatty acid composition of the common jellyfish Aurelia aurita. Mar. Boil. 2001, 138, 1029–1035. [Google Scholar] [CrossRef]
- Ying, C.; Ying, W.; Jing, Z.; Na, W. Potential dietary influence on the stable isotopes and fatty acid compositions of jellyfishes in the Yellow Sea. J. Mar. Boil. Assoc. UK 2012, 92, 1325–1333. [Google Scholar] [CrossRef]
- Wang, M.; MacKenzie, A.D.; Jeffs, A.G. Lipid and fatty acid composition of likely zooplankton prey of spiny lobster (Jasus edwardsii) phyllosomas. Aquac. Nutr. 2015, 21, 385–400. [Google Scholar] [CrossRef]
- Tilves, U.; Fuentes, V.; Milisenda, G.; Parrish, C.; Vizzini, S.; Sabatés, A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: Evidence from stable isotope signatures and fatty acid composition. Mar. Ecol. Prog. Ser. 2018, 591, 101–116. [Google Scholar] [CrossRef]
- Burnett, J.W. Jellyfish Envenomation Syndromes Worldwide; UNEP: Athens, Greece, 1991. [Google Scholar]
- Burnett, J.W. Medical aspects of jellyfish envenomation: Pathogenesis, case reporting and therapy. Hydrobiologia 2001, 451, 1–9. [Google Scholar] [CrossRef]
- Fenner, P.J.; Harrison, S.L. Irukandji and Chironex fleckeri jellyfish envenomation in tropical Australia. Wilderness Environ. Med. 2000, 11, 233–240. [Google Scholar] [CrossRef]
- UNEP. Jellyfish blooms in the Mediterranean. In II Workshop on Jellyfish in the Mediterranean Sea; UNEP, Ed.; UNEP: Athens, Greece, 1991; Volume 47. [Google Scholar]
- Carneiro, R.F.V.; Costa, P.P.C.; Gomes, V.M.; Souza, A.J.F.; Oliveira, S.C.B.; Filho, E.B.S.D.; Zara, F.J.; Fonteles, M.C.; Toyama, D.O.; Toyama, M.H.; et al. The extract of the jellyfish Phyllorhiza punctata promotes neurotoxic effects. J. Appl. Toxicol. 2011, 31, 720–729. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Romano, G. Antipredatory Defensive Role of Planktonic Marine Natural Products. In Handbook of Marine Natural Products; Gerwick, W.H., Ed.; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 711–748. [Google Scholar]
- Hsieh, P.Y.H.; Leong, F.-M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in Southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Morikawa, T. Jellyfish. FAO Infofish Mark. Dig. 1984, 1, 37–39. [Google Scholar]
- Zhang, H.; Zhang, J.Y.; Wang, H.L.; Luo, P.J.; Zhang, J.B. The Revision of Aluminum-containing Food Additive Provisions in China. Biomed. Environ. Sci. 2016, 29, 461–466. [Google Scholar] [PubMed]
- Ozer, N.P.; Celikkale, M.S. Utilization possibilities of jellyfish Rhizostoma pulmo, as a food in the Black Sea. J. Food Sci. Technol. 2001, 38, 175–178. [Google Scholar]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Peggy Hsieh, Y.H.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Radwan, F.F.; Burnett, J.W.; Bloom, D.A.; Coliano, T.; Eldefrawi, M.E.; Erderly, H.; Aurelian, L.; Torres, M.; La Cotera, E.P.H.-D. A comparison of the toxinological characteristics of two Cassiopea and Aurelia species. Toxicon 2001, 39, 245–257. [Google Scholar] [CrossRef]
- Nevalainen, T.J.; Peuravuori, H.J.; Quinn, R.J.; Llewellyn, L.E.; Benzie, J.A.H.; Fenner, P.J.; Winkel, K.D. Phospholipase A2 in Cnidaria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 731–735. [Google Scholar] [CrossRef]
- Bayazit, V. Cytotoxic Effects of Some Animal and Vegetable Extracts and Some Chemicals on Adenohypophyse Carcinoma, Kidney Adenocarcinoma and Skin Carcinoma Cells. J. Med. Sci. 2004, 4, 1–10. [Google Scholar]
- Burnett, J.W.; Calton, G.J.; Larsen, J.B. Significant envenomation by Aurelia aurita, the moon jellyfish. Toxicon 1988, 26, 215–217. [Google Scholar] [CrossRef]
- Benmeir, P.; Rosenberg, L.; Sagi, A.; Vardi, D.; Eldad, A. Jellyfish envenomation: A summer epidemic. Burns 1990, 16, 471–472. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, E.-S.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’Shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P. Recapitulation des Résultats de la Surveillance des Proliférations de Méduses Sur Les côtes Méditerranéennes françAises Durant L’été 1987, Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Athens, Greece, 1991; UNEP, Ed.; UNEP: Athens, Greece, 1991; pp. 51–57. [Google Scholar]
- Kokelj, F.; Del Negro, P.; Tubaro, A. Dermossicità da Chrysaora hysoscella. G. Ital. Dermatol. Venereol. 1989, 124, 297–298. [Google Scholar] [PubMed]
- Burnett, J.W.; Calton, G.J. Venomous pelagic coelenterates: Chemistry, toxicology, immunology and treatment of their stings. Toxicon 1987, 25, 581–602. [Google Scholar] [CrossRef]
- Walker, M. Pharmacological and biochemical properties of a toxin containing material from the jellyfish, Cyanea capillata. Toxicon 1977, 15, 3–14. [Google Scholar] [CrossRef]
- Badre, S. Bioactive toxins from stinging jellyfish. Toxicon 2014, 91, 114–125. [Google Scholar] [CrossRef]
- Helmholz, H.; Ruhnau, C.; Schütt, C.; Prange, A. Comparative study on the cell toxicity and enzymatic activity of two northern scyphozoan species Cyanea capillata (L.) and Cyanea lamarckii (Péron & Léslieur). Toxicon 2007, 50, 53–64. [Google Scholar]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef]
- Quadrifoglio, F.; Avian, M.; Del Negro, P.; Princi, T.; Scuka, M.; Gavinelli, E.; Rottini Sandrini, L. Nematocisti e tossine di Pelagia noctiluca (Forsskål). Nov. Thalass. 1986, 8, 155–162. [Google Scholar]
- Salleo, A.; Calabrese, L.; Barra, D.; La Spada, G. Characterization of protein components of the capsule fluid ad of the capsule wall of the nematocysts of Pelagia noctiluca. Nov. Thalass. 1986, 8, 119–122. [Google Scholar]
- Scarpa, C.; Kokelj, F.; Del Negro, P.; Tubaro, A. Valutazione dell’effetto irritante sulla cute umana di una preparazione di nematocisti di Pelagia noctiluca. Ann. Ital. Derm. Clin. Sper. 1987, 41, 337–341. [Google Scholar]
- Mariottini, G.L.; Giacco, E.; Pane, L. The mauve stinger Pelagia noctiluca (Forsskal, 1775). Distribution, ecology, toxicity and epidemiology of stings. A review. Mar. Drugs 2008, 6, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Marino, A.; La Spada, G.; Pane, L.; Mariottini, G.L. The venom and the toxicity of Pelagia noctiluca (Cnidaria: Scyphozoa). A review of three decades of research in Italian laboratories and future perspectives. J. Biol. Res. Boll. Soc. Ital. Biol. Sper. 2015, 88, 8. [Google Scholar] [CrossRef]
- Rastogi, A.; Sarkar, A.; Chakrabarty, D. Partial purification and identification of a metalloproteinase with anticoagulant activity from Rhizostoma pulmo (Barrel Jellyfish). Toxicon 2017, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cariello, L.; Romano, G.; Spagnuolo, A.; Zanetti, L. Isolation and partial characterization of Rhizolysin, a high molecular weight protein with hemolytic activity, from the jellyfish Rhizostoma pulmo. Toxicon 1988, 26, 1057–1065. [Google Scholar] [CrossRef]
- Allavena, A.; Mariottini, G.L.; Carli, A.; Contini, S.; Martelli, A. In vitro evaluation of the cytotoxic, hemolytic and clastogenic activities of Rhizostoma pulmo toxin(s). Toxicon 1998, 36, 933–936. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Liu, S.; Xing, R.; Guo, Z.; Li, P. Factors affecting the protease activity of venom from jellyfish Rhopilema esculentum Kishinouye. Bioorganic Med. Chem. Lett. 2005, 15, 5370–5374. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Li, R.; Xing, R.; Liu, S.; Li, P. Factors influencing hemolytic activity of venom from the jellyfish Rhopilema esculentum Kishinouye. Food Chem. Toxicol. 2007, 45, 1173–1178. [Google Scholar] [CrossRef]
- Gusmani, L.; Avian, M.; Galil, B.; Patriarca, P.; Rottini, G. Biologically active polypeptides in the venom of the jellyfish Rhopilema nomadica. Toxicon 1997, 35, 637–648. [Google Scholar] [CrossRef]
- Yoffe, B.; Baruchin, A.M. Mediterranean jellyfish (Rhopilema nomadica) sting. Burns 2004, 30, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel Proteinaceous Toxins from the Box Jellyfish (Sea Wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Ratnapala, L.A.; Cooke, I.M.; Yanagihara, A.A. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) venom. Toxicon 2001, 39, 981–990. [Google Scholar] [CrossRef]
- Rottini, G.; Gusmani, L.; Parovel, E.; Avian, M.; Patriarca, P. Purification and properties of a cytolytic toxin in venom of the jellyfish Carybdea marsupialis. Toxicon 1995, 33, 315–326. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, J.; Torrens, E.; Segura-Puertas, L. Partial purification and characterization of a novel neurotoxin and three cytolysins from box jellyfish (Carybdea marsupialis) nematocyst venom. Arch. Toxicol. 2006, 80, 163–168. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Burnell, J.N. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar] [CrossRef]
- Avila Soria, G. Molecular Characterization of Carukia Barnesi and Malo Kingi, Cnidaria; Cubozoa; Carybdeidae; James Cook University: Douglas, Australia, 2009. [Google Scholar]
- Jouiaei, M.; Casewell, N.; Yanagihara, A.; Nouwens, A.; Cribb, B.; Whitehead, D.; Jackson, T.; Ali, S.A.; Wagstaff, S.; Koludarov, I.; et al. Firing the sting: Chemically induced Ddischarge of cnidae reveals novel proteins and peptides from box jellyfish (Chironex fleckeri) Venom. Toxins 2015, 7, 936–950. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Burnell, J.N. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2007, 50, 850–860. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Aziz, A.; Loukas, A.; Potriquet, J.; Seymour, J.; Mulvenna, J. Venom Proteome of the Box Jellyfish Chironex fleckeri. PLoS ONE 2012, 7, e47866. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A Novel Protein Toxin from the Deadly Box Jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vitro effects of two chirodropid (Chironex fleckeri and Chiropsalmus sp.) venoms: Efficacy of box jellyfish antivenom. Toxicon 2003, 41, 703–711. [Google Scholar] [CrossRef]
- Zhang, M.; Fishman, Y.; Sher, D.; Zlotkin, E. Hydralysin, a Novel Animal Group-Selective Paralytic and Cytolytic Protein from a Noncnidocystic Origin in Hydra. Biochemistry 2003, 42, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.J.; Chung, R.; Dunlap, W.C.; Morandini, A.C.; Marques, A.C.; Moura-Da-Silva, A.M.; Ward, M.; Padilla, G.; Da Silva, L.F.; Andreakis, N.; et al. Proteomic characterisation of toxins isolated from nematocysts of the South Atlantic jellyfish Olindias sambaquiensis. Toxicon 2013, 71, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Stillway, L.; Lane, C. Phospholipase in the nematocyst toxin of Physalia physalis. Toxicon 1971, 9, 193–195. [Google Scholar] [CrossRef]
- Tamkun, M.M.; Hessinger, D.A. Isolation and partial characterization of a hemolytic and toxic protein from the nematocyst venom of the Portuguese Man-of-War, Physalia physalis. Biochim. Biophys. Acta BBA Protein Struct. 1981, 667, 87–98. [Google Scholar] [CrossRef]
- Zhuang, Y.-L.; Sun, L.-P.; Zhao, X.; Hou, H.; Li, B.-F. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Hsieh, P.Y.H. Use of Jellyfish Collagen (type II) in the Treatment of Rheumatoid Arthritis. U.S. Patent 6,894,029, 17 May 2005. [Google Scholar]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish (Rhopilema esculentum) on Mice Skin Photoaging Induced by UV Irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Sugahara, T.; Ueno, M.; Goto, Y.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S. Immunostimulation Effect of Jellyfish Collagen. Biosci. Biotechnol. Biochem. 2006, 70, 2131–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimoto, S.; Goto, Y.; Morishige, H.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S.; Sugahara, T. Mode of Action of the Immunostimulatory Effect of Collagen from Jellyfish. Biosci. Biotechnol. Biochem. 2008, 72, 2806–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishige, H.; Sugahara, T.; Nishimoto, S.; Muranaka, A.; Ohno, F.; Shiraishi, R.; Doi, M. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology 2011, 63, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calejo, M.T.; Almeida, A.J.; Fernandes, A.I. Exploring a new jellyfish collagen in the production of microparticles for protein delivery. J. Microencapsul. 2012, 29, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.Y.; Lee, Y.M. Preparation of porous collagen/hyaluronic acid hybrid scaffolds for biomimetic functionalization through biochemical binding affinity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 506–518. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef]
- Nudelman, R.; Alhmoud, H.; Delalat, B.; Fleicher, S.; Fine, E.; Guliakhmedova, T.; Elnathan, R.; Nyska, A.; Voelcker, N.H.; Gozin, M.; et al. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv. Funct. Mater. 2019, 29, 1902783. [Google Scholar] [CrossRef]
- Lau, M.-T.; Manion, J.; Littleboy, J.B.; Oyston, L.; Khuong, T.M.; Wang, Q.-P.; Nguyen, D.T.; Hesselson, D.; Seymour, J.E.; Neely, G.G. Molecular dissection of box jellyfish venom cytotoxicity highlights an effective venom antidote. Nat. Commun. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Mariscal, R.N. Nematocysts. In Coelenterate Biology; Lenhoff, K., Muscatine, L., Marian, E., Davis, L., Eds.; University of Hawaii Press: Honolulu, HI, USA, 1974; pp. 129–178. [Google Scholar]
- Mariottini, G.L.; Pane, L. Mediterranean Jellyfish Venoms: A Review on Scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.-K.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer Effect of Nemopilema nomurai Jellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evid. Based Complement. Altern. Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, P.; Feng, J.; Li, I.; Yu, H. Cytotoxicity of the venom from the nematocysts of jellyfish Cyanea nozakii Kishinouye. Toxicol. Ind. Health 2012, 28, 186–192. [Google Scholar]
- Mariottini, G.L.; Pane, L. Cytotoxic and cytolytic cnidarian venoms. A review on health implications and possible therapeutic applications. Toxins 2014, 6, 108–151. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of cytotoxicity of Pelagia noctiluca venom causes reactive oxygen species generation, lipid peroxydation induction and DNA damage in human colon cancer cells. Lipids Health Dis. 2011, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Bousabbeh, M.; Ben Mabrouk, H.; Morjen, M.; Marrakchi, N.; Bacha, H. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by the Mediterranean jellyfish Pelagia noctiluca venom and its fractions in cultured glioblastoma cells. Lipids Health Dis. 2012, 11, 84. [Google Scholar] [CrossRef]
- Hessinger, D.A.; Lenhoff, H.M. Mechanism of hemolysis induced by nematocyst venom: Roles of phospholipase A and direct lytic factor. Arch. Biochem. Biophys. 1976, 173, 603–613. [Google Scholar] [CrossRef]
- Carli, A.; Mariottini, G.L.; Pane, L. Ecological and Medical Aspects of Jellyfish Poisoning. In Epidemiological Studies Related to the Environmental Quality Criteria for Bathing Waters, Shellfish-Growing Waters and Edible Marine Organisms; MAP Tech. Rep. Ser. 1995, No. 93; UNEP: Athens, Greece, 1995; pp. 1–21. [Google Scholar]
- Ayed, Y.; Dellai, A.; Ben Mansour, H.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef]
- Morabito, R.; Condello, S.; Curro, M.; Marino, A.; Ientile, R.; La Spada, G. Oxidative stress induced by crude venom from the jellyfish Pelagia noctiluca in neuronal-like differentiated SH-SY5Y cells. Toxicol. Vitr. 2012, 26, 694–699. [Google Scholar] [CrossRef]
- Morabito, R.; La Spada, G.; Crupi, R.; Esposito, E.; Marino, A. Crude venom from nematocysts of the jellyfish Pelagia noctiluca as a tool to study cell physiology. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 8. [Google Scholar] [CrossRef]
- Steinberger, L.R.; Gulakhmedova, T.; Barkay, Z.; Gozin, M.; Richter, S. Jellyfish-based plastic. Adv. Sustain. Syst. 2019, 3, 1900016. [Google Scholar] [CrossRef]

| Species | Tissue | Proteins | Lipids | Carbohydrates | Proteins | Lipids | Carbohydrates | References |
|---|---|---|---|---|---|---|---|---|
| (% Medusa WM) | (% Medusa DM) | |||||||
| Semaeostomeae | ||||||||
| Aurelia aurita | W | 0.5 | 0.03 | [24] | ||||
| W | 0.4 | [25] | ||||||
| W | 0.2 | [26] | ||||||
| W | 4.7 | 9.2 | 13.5 | 5.3 | 2 | 3.4 | [27] | |
| W | 5.9 | 1.9 | 2.9 | [28] | ||||
| G | 23.7 | 14.6 | [28] | |||||
| OA | 7.3 | 2.6 | [28] | |||||
| B | 4.2 | 1.5 | [28] | |||||
| W | 2.1–28.6 | 1.2–3.4 | 0.4–1.1 | [29] | ||||
| G | 4.4–23.0 | 2.6–6.0 | 1.1–2.1 | [29] | ||||
| OA | 4.1–15.3 | 1.3–4.0 | 0.6–1.5 | [29] | ||||
| B | 2.3–8.3 | 0.9–2.9 | 0.3–0.9 | [29] | ||||
| W | 0.03 | [30] | ||||||
| W | 0.7 | 0.04 | [30] | |||||
| W | 3.5 | 0.4 | 19.9 | [31] | ||||
| Aurelia sp.1 | W | 5.7 | 4.1 | [32] | ||||
| Chrysaora hysoscella | W | 2.7 | [26] | |||||
| C. pacifica | W | 7.5 | 0.7 | 22.7 | [31] | |||
| C. quinquecirrha | W | 0.2 | [24] | |||||
| MG | 6.1 | [24] | ||||||
| FG | 5.5 | [24] | ||||||
| T | 4.1 | [24] | ||||||
| Cyanea capillata | W | 16.5 | 0.5 | 0.9 | [33] | |||
| G | 28.4 | 0.6 | 0.9 | [33] | ||||
| OA | 29.8 | 1.2 | 1.1 | [33] | ||||
| B | 7.9 | 0.2 | 0.8 | [33] | ||||
| G | 9.6 | 1.6 | 1 | [34] | ||||
| W | 0.3–0.8 | [35] | ||||||
| C. lamarckii | W | 0.7 | [26] | |||||
| Pelagia noctiluca | W | 10.9–19.8 | 1.3–2.9 | 0.1–0.7 | [36,37] | |||
| W | 0.2 | [38] | ||||||
| Poralia rufescens | W | 0.2 | 0.4 | 0.1 | [34] | |||
| Stygiomedusa gigantea | W | 10.2 | 0.5 | [39] | ||||
| Rhizostomeae | ||||||||
| Acromitus maculosus | OA | 33.7 | 1.1 | 6 | 1.3 | [40] | ||
| B | 21.4 | 0.4 | 17.7 | 0.8 | [40] | |||
| Catostylus tagi | W | 0.4 | [41] | |||||
| OA | 4.3 | 0.5 | [41] | |||||
| B | 1.8 | 0.2 | [41] | |||||
| Cotylorhiza tuberculata | W | 2.2 | 12.3 | [32] | ||||
| G | 36.8 | 6 | [42] | |||||
| OA | 20 | 6.4 | [42] | |||||
| B | 12 | 0.7 | [42] | |||||
| BM | 7.6 | 0.5 | [42] | |||||
| Rhizostoma octopus | W | 12.8 | 0.3 | 0.8 | [33] | |||
| G | 12.1 | 0.6 | 0.9 | [33] | ||||
| OA | 13.4 | 0.3 | 0.7 | [33] | ||||
| B | 6.6 | 0.3 | 0.7 | [33] | ||||
| R. pulmo | W | 2.3 | [43] | |||||
| W | 6 | 4 | [32] | |||||
| G | 18 | 1.2 | [42] | |||||
| OA | 27 | 0.8 | [42] | |||||
| B | 8.7 | 0.7 | [42] | |||||
| BM | 13.7 | 1 | [42] | |||||
| R. luteum | W | 0.8–1.9 | [44] | |||||
| Rhopilema hispidum | OA | 43.8 | 1.4 | 10.7 | 2 | [40] | ||
| B | 19.9 | 0.5 | 18.2 | 0.5 | [40] | |||
| R. esculentum | OA | 53.9 | 1.8 | 7.7 | 2.8 | [40] | ||
| B | 38.1 | 0.6 | 8.9 | 1.6 | [40] | |||
| Stomolophus meleagris | B | 1.1 | [45] | |||||
| M | 1 | [45] | ||||||
| Coronatae | ||||||||
| Atolla wyvillei | W | 1.1 | [46] | |||||
| W | 16.9 | 4.2 | 1.7 | 0.8 | 0.2 | 0.1 | [47] | |
| W | 0.3 | 0.01 | [39] | |||||
| Collagen Type | Tissue |
|---|---|
| I | bone, dermis, tendon, ligaments, cornea |
| II | cartilage, vitreous body, nucleus pulposus |
| III | skin, vessel walls, reticular fibres of most tissues (lungs, liver, spleen) |
| IV | basement membranes |
| VI | cornea (often associated with type I collagen) |
| Collagen Type | Species | Tissue | Collagen Content | References | |
|---|---|---|---|---|---|
| Pepsin | Acid | ||||
| I | Priacanthus tayenus | Bone | 1.6 | [52] | |
| Skin | 10.9 | [52] | |||
| Mystus macropterus | Skin | 28.0 | 16.8 | [53] | |
| Syngnathus schlegeli | Skin | 33.2 | 5.5 | [54] | |
| Lagocephalus gloveri | Skin | 54.3 | [55] | ||
| Takifugu rubripes | Skin | 44.7 | 10.7 | [56] | |
| Saurida spp. | Scales | 0.79 | [57] | ||
| Trachurus japonicus | Scales | 1.5 | [57] | ||
| Mugil cephalis | Scales | 0.4 | [57] | ||
| Cypselurus melanurus | Scales | 0.7 | [57] | ||
| Dentex tumifrons | Scales | 0.9 | [57] | ||
| Illex argentinus | Skin | 53 | [58] | ||
| Sepiella inermis | Skin | 16.2 | 0.6 | [59] | |
| II | Chiloscyllium punctatum | Cartilage | 9.6 | 1.3 | [60] |
| Carcharhinus limbatus | Cartilage | 10.3 | 1.0 | [60] | |
| IV | Marine sponge | 30 | [51,61] | ||
| Species | Tissue | Collagen Content | References | |||
|---|---|---|---|---|---|---|
| Pepsin | Acid | |||||
| (% DM) | (% WM) | (% DM) | (% WM) | |||
| Aurelia aurita | W | 0.01 | [64] | |||
| Chrysaora sp. | B | 9–19 | [65] | |||
| Pelagia noctiluca | W | 0.07 | [64] | |||
| Catostylus tagi | B | 2.7 | [66] | |||
| Cotylorhiza tuberculata | B | 4.5 | [64] | |||
| OA | 19.4 | [64] | ||||
| B | <10 | [64] | ||||
| Rhizostoma pulmo | B | 8.3–31.5 | [64] | |||
| OA | 26–90 | [64] | ||||
| B | <10 | [64] | ||||
| Rhopilema asamushi | - | 35.2 | [67] | |||
| Rhopilema esculentum | M | 0.28 | 0.12 | [68] | ||
| Stomolophus meleagris | M | 46.4 | [69] | |||
| Nemopilema nomurai | M | 2.2 | [70] | |||
| Semaeostomeae | Rhizostomeae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Aurelia sp. | Aurelia aurita | Chrysaora sp. | Chrysaora hysoscella | Chrysaora Pacifica | Pelagia noctiluca | Catostylus Tagi | Cotylorhiza Tuberculata | Rhizostoma Pulmo | Rhopilema esculentum | Stomolophus Meleagris | Nemopilema Nomurai | |||
| * | ** | ** | * | ||||||||||||
| Tissue | W | W | W | W | W | W | W | B | OA | W | W | W | W | W | W |
| Amino-acids | |||||||||||||||
| Hydroxyproline | - | - | 70 | - | - | - | 65 | 21.9 | 16.9 | - | - | - | - | 40 | 57 |
| Aspartic acid | 20 | 94 | 76 | 12.2 | 86 | 6.9 | 84 | 97.5 | 98.4 | 25 | 32 | 8.4 | 68 | 79 | 71 |
| Serine | 60 | 46 | 44 | 6.2 | 46 | 2.9 | 42 | 48.2 | 50.3 | 55 | 67 | 3.9 | 44 | 45 | 45 |
| Glutamic acid | 87 | 138 | 101 | 17.6 | 139 | 10.3 | 115 | 141.3 | 152.2 | 160 | 152 | 12.9 | 86 | 98 | 94 |
| Glycine | 352 | 145 | 320 | 19.6 | 166 | 13.5 | 269 | 94.2 | 89.3 | 59 | 53 | 8.4 | 268 | 309 | 344 |
| Histidine | n.d. | 12 | n.d. | 2.5 | 14 | 0.9 | - | 8.8 | 12 | 78 | 56 | 1.4 | 6 | 2 | 1 |
| Arginine | 7 | 69 | 58 | 8.3 | 64 | 5 | 62 | 77.7 | 68.7 | - | 20 | 6.4 | 77 | 52 | 57 |
| Threonine | 64 | 50 | 34 | 6 | 45 | 3.1 | 31 | 48.2 | 46.3 | 74 | 50 | 4.3 | 36 | 35 | 28 |
| Alanine | 45 | 67 | 87 | 6.5 | 66 | 4.1 | 101 | 70.1 | 64.7 | 43 | 39 | 4.7 | 109 | 82 | 77 |
| Proline | 27 | 104 | 79 | 6.2 | 107 | 4.1 | 78 | 75.6 | 68.1 | 51 | 39 | 5.1 | 72 | 82 | 79 |
| Cystine | 26 | 5 | n.d. | - | 4 | - | 1 | 12 | 10.9 | - | 13 | - | 3 | - | - |
| Tyrosine | 60 | 29 | 10 | 4.6 | 30 | 1.8 | 4 | 28.5 | 31.5 | 70 | 76 | 2.6 | 18 | 6 | 5 |
| Valine | 43 | 36 | 22 | 6 | 36 | 3.1 | 24 | 44.9 | 46.3 | 59 | 49 | 4.3 | 38 | 35 | 24 |
| Methionine | 38 | 15 | 16 | - | 19 | - | 5 | 18.6 | 19.5 | 53 | 46 | - | 12 | 4 | 8 |
| Lysine | 60 | 68 | 17 | 10.4 | 64 | 4.9 | 29 | 72.3 | 76.7 | 61 | 69 | 7 | 51 | 38 | 24 |
| Isoleucine | 43 | 32 | 23 | 5.5 | 33 | 2.6 | 22 | 36.1 | 37.2 | 57 | 55 | 3.5 | 31 | 22 | 16 |
| Leucine | n.d. | 44 | 31 | 7.8 | 56 | 3.6 | 31 | 56.9 | 62.4 | 74 | 91 | 5.1 | 42 | 34 | 27 |
| Phenylalanine | 66 | 44 | 14 | 5.3 | 25 | 2.1 | 6 | 36.1 | 42.3 | 80 | 93 | 3.3 | 30 | 10 | 8 |
| Hydroxylysine | - | - | - | - | - | - | 32 | 11.2 | 6.3 | - | - | - | - | 27 | 35 |
| Tryptophan | n.d. | - | - | - | - | - | - | - | - | n.d. | n.d. | - | 0 | 0 | 0 |
| Reference | [32] | [31] | [65] | [23] | [31] | [23] | [66] | [66] | [66] | [32] | [32] | [23] | [68] | [69] | [70] |
| Species | Tissue | Total SFAs | Total MUFAs | Total PUFAs | ω-3 | ω-6 | ω-6/ω-3 | Location | References |
|---|---|---|---|---|---|---|---|---|---|
| Semaeostomeae | |||||||||
| Aurelia aurita | W | 29.4 | 37.1 | 27.9 | 18 | 10 | 0.6 | NW Atlantic | [79] |
| W | 54 | 13.8 | 32.3 | 26.3 | 5.8 | 0.2 | Irish Sea | [80] | |
| W | 46.7 | 19.2 | 28 | 20.9 | 7.1 | 0.4 | Seto Inland Sea | [81] | |
| W | 29.8.1 | 12 | 57.2 | 38.5 | 18.7 | 0.5 | Yellow Sea | [82] | |
| W | 41 | 8.4 | 33.4 | 11.3 | 16.9 | 0.7 | New Zealand | [83] | |
| W | 53.4.3 | 12.2 | 30.7 | Tokyo Bay | [31] | ||||
| W | 76.7 | 15.3 | 3.9 | Ionian Sea | [30] | ||||
| Aurelia sp.1 | W | 69.5 | 4.7 | 25.8 | 19 | 6.8 | 0.4 | NW Mediterranean | [32] |
| Chrysaora hysoscella | W | 22.7 | 22.4 | 55 | 47.1 | 6.2 | 0.1 | Irish Sea | [26] |
| Chrysaora pacifica | W | 45.9 | 13 | 35.3 | Tokyo Bay | [31] | |||
| Chrysaora quinquecirrha | W | 23.5 | 8.2 | 59.5 | 23.6 | 35.9 | 1.5 | Charleston harbour | [24] |
| Cyanea lamarckii | W | 40.2 | 19.2 | 40.8 | 30.1 | 9.1 | 0.3 | Irish Sea | [26] |
| Cyanea capillata | W | 26.1 | 23.3 | 47.4 | 34.6 | 12.7 | 0.4 | NW Atlantic | [35] |
| Cyanea nozakii | W | 29.9 | 6 | 57.9 | 26.9 | 30.5 | 1.1 | Yellow Sea | [82] |
| Pelagia noctiluca (medusae) | W | 63.4 | 21.1 | 10.2 | 4.8 | 3.8 | 0.8 | NW Mediterranean | [84] |
| Pelagia noctiluca (ephyrae) | W | 33 | 11 | 52.1 | 40.6 | 10.7 | 0.3 | NW Mediterranean | [84] |
| Stygiomedusa gigantea | W | 24.2 | 41.3 | 31 | 28.5 | 2.5 | 0.1 | Antarctic | [39] |
| Rhizostomeae | |||||||||
| Cotylorhiza tuberculata | W | 54.8 | 15.2 | 30 | 16.4 | 13.6 | 0.8 | NW Mediterranean | [32] |
| Rhizostoma luteum | W | 30.2 | 20.8 | 49 | 15.6 | 33.4 | 2.1 | NW Mediterranean | [44] |
| Rhizostoma octopus | W | 59.8 | 15.3 | 25.1 | 20.7 | 4.4 | 0.2 | Irish Sea | [26] |
| Rhizostoma pulmo | W | 68.2 | 7 | 24.8 | 13.5 | 11.3 | 0.8 | NW Mediterranean | [32] |
| Stomolophus meleagris | W | 23 | 6.8 | 59.9 | 39.7 | 20.2 | 0.5 | Charleston harbour | [24] |
| B | 36.8 | 6.4 | 56.8 | 38.2 | 18.4 | 0.5 | Yellow Sea | [82] | |
| OA | 35.6 | 4.5 | 59.9 | 38.1 | 21.3 | 0.6 | Yellow Sea | [82] | |
| Coronatae | |||||||||
| Atolla wyvillei | W | 30.9 | 30.6 | 34.2 | 31.1 | 3.1 | 0.2 | Antarctic | [39] |
| Species | Venom Main Component | Molecular Mass (kDa) | Biological Activity | References |
|---|---|---|---|---|
| Scyphozoa | ||||
| Aurelia aurita | Phospholipase A2 | Cytolytic Hemolytic, neurotoxic, myotoxic, local skin irritation | [98,99] | |
| Proteolytic enzymes | [100] | |||
| Tetramine and unidentified protein | Dermotoxic, temporary paralysis, oedema | [101,102] | ||
| TX-1 | 54 | [103] | ||
| TX-2 | 51 | [103] | ||
| Metalloproteinases | Gelatinolytic, caseinolytic, fibrinolytic | [104] | ||
| Aurelin | 4.30 | [105] | ||
| Cassiopea andromeda | Phospholipase A2 | Hemolytic, dermonecrotic, local skin irritation | [98] | |
| C. xamancha | Phospholipase A2 | Hemolytic, dermonecrotic, local skin irritation | [98] | |
| Cotylorhiza tuberculata | Unharmful | [106] | ||
| Chrysaora hysoscella | Cationic protein | Dermotoxic, cytotoxic | [107] | |
| C. quinquecirrha | DNase | 110 | Dermonecrotic, cytotoxic | [108] |
| Acid protease | 120–150 | [108] | ||
| Alkaline protease (metallopeptidase) | 100 | [108] | ||
| Collagenase | [108] | |||
| Cyanea capillata | Basic protein(s) | 70 | Cardiotoxic, dermonecrotic, musculotoxic | [108,109] |
| CcTX-1 | 31.173 | Cytotoxic | [110] | |
| CcNT | 8.22 | Neurotoxic | [110] | |
| Phospholipase A2 | Cytolytic, cytotoxic, hemolytic | [99,111] | ||
| C. lamarckii | ClGP-1 | 27 | Cytotoxic | [110] |
| Phospholipase A2 | Cytolytic, cytotoxic, hemolytic | [111] | ||
| C. nozakii | Metalloproteinases | Gelatinolytic, caseinolytic, fibrinolytic | [104] | |
| Nemopilema nomurai | Metalloproteinases | 28–36 | Gelatinolytic, caseinolytic, fibrinolytic | [104] |
| 20–40/10–15 | Cytotoxic, hemolytic | [112] | ||
| Pelagia noctiluca | Proteinaceous macromolecules | 44–66 | Hemolytic, cytotoxic, dermonecrotic, hemolytic, local tissue damage | [113,114,115,116,117] |
| Phyllorhiza punctata | Phospholipase A2 Saxitoxin * Gonyautoxin-4 * Tetrodotoxin * Brevetoxin-2 * | Neurotoxic | [89] | |
| Rhizostoma pulmo | Rhizoprotease | 95 | Proteolytic, hemolytic | [118] |
| Rhizolysin | 260 | Hemolytic | [119] | |
| Cytotoxic, hemolytic | [120] | |||
| Rhopilema esculentum | Metalloproteinases | Gelatinolytic, caseinolytic, fibrinolytic | [104] | |
| Hyaluronidase | 55–95 | Degradation of extracellular matrix components | [104] | |
| Proteolytic, cytotoxic, hemolytic | [121,122] | |||
| R. nomadica | Phospholipase A2 | Hemolytic | [123] | |
| Serine protease | Local skin damage | [124] | ||
| Rhopilema sp. | Phospholipase A2 | Hemolytic | [110] | |
| Stomolophus meleagris | SmP90 | 90 | Radical scavenging | [110] |
| Phospholipase A2, C-lectin, ShK, Kv+ toxin, Metalloproteinases | Cytotoxic, cytolytic, hemolytic, local tissue damage | [125] | ||
| Cubozoa | ||||
| Alatina moseri | CaTX-A | 43 | Hemolytic | [103] |
| Carybdea alata | CaTX-A (CAH1) | 43 | Hemolytic Hemolytic | [110,126,127] |
| CaTX-B | 45 | |||
| C. marsupialis | Haemolysin | 102–107 | Cytolytic Hemolytic Hemolytic | [110,128,129] |
| CmHl5 | 220 | |||
| CmHl1 | 139 | |||
| CmHl7 | 36 | |||
| CmNt | 120 | Neurotoxic, hemolytic | [129] | |
| C. rastonii | Phospholipase A2 | Cytolytic Hemolytic Hemolytic | [99] | |
| CrTX-II | [130] | |||
| CrTX-III | [130] | |||
| CrTX-A | 43 | [103,110,126] | ||
| CrTX-B | 46 | [110,126] | ||
| Carukia barnesi | Phospholipase A2 | Cytolytic, hemolytic | [99] | |
| CbTX-I | 21.67 | Neurotoxic | [131] | |
| CbTX-II | 18.16 | Neurotoxic | [131] | |
| Chironex fleckeri | Phospholipase A2 | Cytolytic, hemolytic | [99] | |
| Metalloproteinases | 17–130 | [132] | ||
| CfTX-1 | 43 | Cardiotoxic, cytotoxic, dermonecrotic, lethal | [103,110,132,133,134] | |
| CfTX-2 | 45 | |||
| CfTX-A | 40 | |||
| CfTX-B | 42 | |||
| CfTX-Bt | 31.293 | [103] | ||
| Chiropsalmus quadrigatus | CqTX-A | 44 | Hemolytic, neurotoxic, myotoxic | [110,135,136] |
| Malo kingi | MkTX-A | 48.55 | Dermonecrotic, inflammatory | [131] |
| MkTX-B | ||||
| Hydrozoa | ||||
| Hydra magnipapillata | CqTX-A | [103] | ||
| H. viridissima | Hydralysin | 27 | Neurotoxic, cytolytic, paralytic | [137] |
| Millepora sp. | Phospholipase A2 | Cytolytic, hemolytic | [99] | |
| Obelia geniculata | Phospholipase A2 | Cytolytic, hemolytic | [99] | |
| Olindias sambaquiensis | Oshem1 | 3.013 | Hemolytic Hemolytic | [110] |
| Oshem2 | 3.376 | [110] | ||
| Metalloproteinases | Cytolytic, neurotoxic | [138] | ||
| Physalia physalis | Phospholipase A2 | [139] | ||
| Phospholipase B | [139] | |||
| Physalitoxin | 220 | Hemolytic | [110,140] | |
| P1 | 220 | Neurotoxic Neurotoxic | [110] | |
| P3 | 85 | [110] | ||
| PpV9.4 | 0.55 | Hemolytic Neurotoxic, cardiotoxic | [110] | |
| PpV19.3 | 4.72 | [110] | ||
| Elastase Histamine | Musculotoxic, cytolytic, hemolytic | [108] | ||
| Collagenase | 25 | Cytolytic, hemolytic | [108] | |
| DNase | 75 | [108] | ||
| Tubularia larynx | Phospholipase A2 | Cytolytic, hemolytic | [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. https://doi.org/10.3390/md17110604
Merquiol L, Romano G, Ianora A, D’Ambra I. Biotechnological Applications of Scyphomedusae. Marine Drugs. 2019; 17(11):604. https://doi.org/10.3390/md17110604
Chicago/Turabian StyleMerquiol, Louise, Giovanna Romano, Adrianna Ianora, and Isabella D’Ambra. 2019. "Biotechnological Applications of Scyphomedusae" Marine Drugs 17, no. 11: 604. https://doi.org/10.3390/md17110604
APA StyleMerquiol, L., Romano, G., Ianora, A., & D’Ambra, I. (2019). Biotechnological Applications of Scyphomedusae. Marine Drugs, 17(11), 604. https://doi.org/10.3390/md17110604