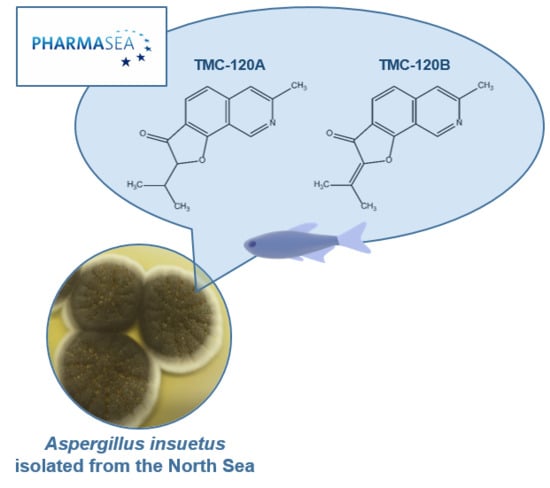
Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B
Abstract
:1. Introduction
2. Results and Discussion
2.1. Zebrafish-Based Antiseizure Drug Discovery
2.2. Bioactivity-Guided Purification of Active Compounds
2.3. TMC-120A, TMC-120B, and Structural Analogues Ameliorate Seizures in the Zebrafish PTZ Seizure Model
2.4. TMC-120A and TMC-120B Ameliorate Epileptiform Brain Activity in the Zebrafish PTZ Seizure Model
2.5. TMC-120A and TMC-120B Ameliorate Focal Seizures in the Mouse 6-Hz (44 mA) Psychomotor Seizure Model
3. Materials and Methods
3.1. General Chemical Experimental Procedures
3.2. Fungal Strains
3.3. Cultivation
3.4. Extraction and Isolation
3.5. Compound and Sample Preparation
3.6. Experimental Animals
3.6.1. Zebrafish
3.6.2. Mice
3.7. Zebrafish Photomotor Response Assay
3.7.1. Behavioral Analysis
3.7.2. Toxicity Evaluation
3.8. Zebrafish Pentylenetetrazole Seizure Model
3.8.1. Toxicity Evaluation
3.8.2. Behavioral Analysis
3.8.3. Electrophysiology
3.9. Mouse 6-Hz (44 mA) Psychomotor Seizure Model
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from Marine Sources: An Assessment of the Current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Martinez Arbizu, P.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef]
- Copmans, D.; Rateb, M.; Tabudravu, J.N.; Perez-Bonilla, M.; Dirkx, N.; Vallorani, R.; Diaz, C.; Perez Del Palacio, J.; Smith, A.J.; Ebel, R.; et al. Zebrafish-Based Discovery of Antiseizure Compounds from the Red Sea: Pseurotin A2 and Azaspirofuran A. ACS Chem. Neurosci. 2018, 9, 1652–1662. [Google Scholar] [CrossRef]
- Sander, J.W. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 2003, 16, 165–170. [Google Scholar] [CrossRef]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Singh, A.; Trevick, S. The Epidemiology of Global Epilepsy. Neurol. Clin. 2016, 34, 837–847. [Google Scholar] [CrossRef]
- Fisher, R.S.; Van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Golyala, A.; Kwan, P. Drug development for refractory epilepsy: The past 25 years and beyond. Seizure 2017, 44, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalic, L.; Cook, M.J. Managing drug-resistant epilepsy: Challenges and solutions. Neuropsychiatr. Dis. Treat. 2016, 12, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Blond, B.N.; Detyniecki, K.; Hirsch, L.J. Assessment of Treatment Side Effects and Quality of Life in People with Epilepsy. Neurol. Clin. 2016, 34, 395–410. [Google Scholar] [CrossRef]
- Moshe, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Cramer, J.A.; Mintzer, S.; Wheless, J.; Mattson, R.H. Adverse effects of antiepileptic drugs: A brief overview of important issues. Expert Rev. Neurother. 2010, 10, 885–891. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Simonato, M.; French, J.A.; O’Brien, T.J. Joint AES/ILAE translational workshop to optimize preclinical epilepsy research. Epilepsia 2013, 54 (Suppl. 4), 1–2. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshe, S.L.; Perucca, E.; Engel, J., Jr.; Loscher, W.; Noebels, J.L.; Pitkanen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef]
- Loscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef]
- Barker-Haliski, M.L.; Johnson, K.; Billingsley, P.; Huff, J.; Handy, L.J.; Khaleel, R.; Lu, Z.; Mau, M.J.; Pruess, T.H.; Rueda, C.; et al. Validation of a Preclinical Drug Screening Platform for Pharmacoresistant Epilepsy. Neurochem. Res. 2017, 42, 1904–1918. [Google Scholar] [CrossRef]
- Rihel, J.; Schier, A.F. Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 2012, 72, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Lidster, K.; Jefferys, J.G.; Blumcke, I.; Crunelli, V.; Flecknell, P.; Frenguelli, B.G.; Gray, W.P.; Kaminski, R.; Pitkanen, A.; Ragan, I.; et al. Opportunities for improving animal welfare in rodent models of epilepsy and seizures. J. Neurosci. Methods 2016, 260, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grone, B.P.; Baraban, S.C. Animal models in epilepsy research: Legacies and new directions. Nat. Neurosci. 2015, 18, 339–343. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.D.; Esguerra, C.V.; De Witte, P.A. Fishing for drugs from nature: Zebrafish as a technology platform for natural product discovery. Planta Med. 2008, 74, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Kokel, D.; Dunn, T.W.; Ahrens, M.B.; Alshut, R.; Cheung, C.Y.; Saint-Amant, L.; Bruni, G.; Mateus, R.; Van Ham, T.J.; Shiraki, T.; et al. Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 3834–3843. [Google Scholar] [CrossRef]
- Copmans, D.; Meinl, T.; Dietz, C.; Van Leeuwen, M.; Ortmann, J.; Berthold, M.R.; De Witte, P.A. A KNIME-Based Analysis of the Zebrafish Photomotor Response Clusters the Phenotypes of 14 Classes of Neuroactive Molecules. J. Biomol. Screen. 2016, 21, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Mandhane, S.N.; Aavula, K.; Rajamannar, T. Timed pentylenetetrazol infusion test: A comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure 2007, 16, 636–644. [Google Scholar] [CrossRef]
- Copmans, D.; Siekierska, A.; De Witte, P.A.M. Zebrafish Models of Epilepsy and Epileptic Seizures. In Models of Seizures and Epilepsy, 2nd ed.; Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., Moshé, S.L., Eds.; Elsevier: London, UK, 2017; pp. 369–384. [Google Scholar]
- Afrikanova, T.; Serruys, A.S.; Buenafe, O.E.; Clinckers, R.; Smolders, I.; De Witte, P.A.; Crawford, A.D.; Esguerra, C.V. Validation of the zebrafish pentylenetetrazol seizure model: Locomotor versus electrographic responses to antiepileptic drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Due, M.; Varga, J.; Meijer, M.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 2007, 59, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Kildgaard, S.; Mansson, M.; Dosen, I.; Klitgaard, A.; Frisvad, J.C.; Larsen, T.O.; Nielsen, K.F. Accurate dereplication of bioactive secondary metabolites from marine-derived fungi by UHPLC-DAD-QTOFMS and a MS/HRMS library. Mar. Drugs 2014, 12, 3681–3705. [Google Scholar] [CrossRef] [PubMed]
- Laatsch, H. Wiley-VCH: Weinheim. Available online: http://www.wileyvch.de/stmdata/antibase.php (accessed on 1 November 2016).
- Kohno, J.; Hiramatsu, H.; Nishio, M.; Sakurai, M.; Okuda, T.; Komatsubara, S. Structures of TMC-120A, B and C, novel isoquinoline alkaloids from Aspergillus ustus TC 1118. Tetrahedron 1999, 55, 11247–11252. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, Y.; Miao, C.D.; Liu, P.P.; Hong, K.; Zhu, W.M. Sesquiterpenoids and Benzofuranoids from the Marine-Derived Fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Boonyuen, N.; Rachtawee, P.; Laksanacharoen, P.; Pittayakhajonwut, P. Penicisochromans from the endophytic fungus Penicillium sp BCC18034. Phytochem. Lett. 2014, 10, 13–18. [Google Scholar] [CrossRef]
- Slack, G.J.; Puniani, E.; Frisvad, J.C.; Samson, R.A.; Miller, J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009, 113, 480–490. [Google Scholar] [CrossRef]
- Isgut, M.; Rao, M.; Yang, C.; Subrahmanyam, V.; Rida, P.C.G.; Aneja, R. Application of Combination High-Throughput Phenotypic Screening and Target Identification Methods for the Discovery of Natural Product-Based Combination Drugs. Med. Res. Rev. 2018, 38, 504–524. [Google Scholar] [CrossRef]
- Kohno, J.; Sakurai, M.; Kameda, N.; Nishio, M.; Kawano, K.; Kishi, N.; Okuda, T.; Komatsubara, S. Production, isolation and biological properties of TMC-120A, B and C, novel inhibitors of eosinophil survival from Aspergillus ustus TC 1118. J. Antibiot. 1999, 52, 913–916. [Google Scholar] [CrossRef]
- Miller, J.D.; Sun, M.; Gilyan, A.; Roy, J.; Rand, T.G. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem.-Biol. Interact. 2010, 183, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.G.; Dipenta, J.; Robbins, C.; Miller, J.D. Effects of low molecular weight fungal compounds on inflammatory gene transcription and expression in mouse alveolar macrophages. Chem. Biol. Interact. 2011, 190, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.W.; Frey, L.C.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; St. Louis, E.K. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; St. Louis, E.K., Frey, L.C., Eds.; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Zdebik, A.A.; Mahmood, F.; Stanescu, H.C.; Kleta, R.; Bockenhauer, D.; Russell, C. Epilepsy in kcnj10 morphant zebrafish assessed with a novel method for long-term EEG recordings. PLoS ONE 2013, 8, e79765. [Google Scholar] [CrossRef] [PubMed]
- Copmans, D.; Orellana-Paucar, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Foubert, K.; Exarchou, V.; Siekierska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated flavonoids as anti-seizure agents: Naringenin 4’,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, K.S.; Dixon-Salazar, T.; Sills, G.J.; Ben-Menachem, E.; White, H.S.; Porter, R.J.; Dichter, M.A.; Moshe, S.L.; Noebels, J.L.; Privitera, M.D.; et al. Issues related to development of new antiseizure treatments. Epilepsia 2013, 54 (Suppl. 4), 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, M.J.; Dean, R.S. Psychomotor Seizures. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 1191–1192. [Google Scholar] [CrossRef]
- Buenafe, O.E.; Orellana-Paucar, A.; Maes, J.; Huang, H.; Ying, X.; De Borggraeve, W.; Crawford, A.D.; Luyten, W.; Esguerra, C.V.; De Witte, P. Tanshinone IIA exhibits anticonvulsant activity in zebrafish and mouse seizure models. ACS Chem. Neurosci. 2013, 4, 1479–1487. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M.; Afrikanova, T.; Thomas, J.; Aibuldinov, Y.K.; Dehaen, W.; De Witte, P.A.; Esguerra, C.V. Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS ONE 2013, 8, e81634. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M.; Serruys, A.S.; Afrikanova, T.; Maes, J.; De Borggraeve, W.; Alen, J.; Leon-Tamariz, F.; Wilches-Arizabala, I.M.; Crawford, A.D.; De Witte, P.A.; et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012, 24, 14–22. [Google Scholar] [CrossRef]
- Gram, L.; Melchiorsen, J.; Bruhn, J.B. Antibacterial Activity of Marine Culturable Bacteria Collected from a Global Sampling of Ocean Surface Waters and Surface Swabs of Marine Organisms. Mar. Biotechnol. 2010, 12, 439–451. [Google Scholar] [CrossRef]
- Frisvad, J.C. Fungal Secondary Metabolism—Methods and Protocols; Keller, N.P., Turner, G., Eds.; Humana Press: New York, NY, USA, 2012; Volume 944, pp. 47–58. [Google Scholar]









| IBT Number | Species |
|---|---|
| 4133 | Aspergillus ustus |
| 10619 | Aspergillus ustus |
| 28485 | Aspergillus insuetus |
| 914826 | Aspergillus calidoustus |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copmans, D.; Kildgaard, S.; Rasmussen, S.A.; Ślęzak, M.; Dirkx, N.; Partoens, M.; Esguerra, C.V.; Crawford, A.D.; Larsen, T.O.; de Witte, P.A.M. Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B. Mar. Drugs 2019, 17, 607. https://doi.org/10.3390/md17110607
Copmans D, Kildgaard S, Rasmussen SA, Ślęzak M, Dirkx N, Partoens M, Esguerra CV, Crawford AD, Larsen TO, de Witte PAM. Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B. Marine Drugs. 2019; 17(11):607. https://doi.org/10.3390/md17110607
Chicago/Turabian StyleCopmans, Daniëlle, Sara Kildgaard, Silas A. Rasmussen, Monika Ślęzak, Nina Dirkx, Michèle Partoens, Camila V. Esguerra, Alexander D. Crawford, Thomas O. Larsen, and Peter A. M. de Witte. 2019. "Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B" Marine Drugs 17, no. 11: 607. https://doi.org/10.3390/md17110607
APA StyleCopmans, D., Kildgaard, S., Rasmussen, S. A., Ślęzak, M., Dirkx, N., Partoens, M., Esguerra, C. V., Crawford, A. D., Larsen, T. O., & de Witte, P. A. M. (2019). Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B. Marine Drugs, 17(11), 607. https://doi.org/10.3390/md17110607