Fragilides U–W: New 11,20-Epoxybriaranes from the Sea Whip Gorgonian Coral Junceella fragilis
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. In Vitro Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bayer, F.M.; Grasshoff, M. The genus group taxa of the family Ellisellidae, with clarification of the genera established by J.E. Gray (Cnidaria: Octocorallia). Senckenb. Biol. 1994, 74, 21–45. [Google Scholar]
- Chen, C.-C.; Chang, K.-H. Gorgonacea (Coelenterata: Anthozoa: Octocorallia) of Southern Taiwan. Bull. Inst. Zool. Acad. Sin. 1991, 30, 149–181. [Google Scholar]
- Chung, H.-M.; Wang, Y.-C.; Tseng, C.-C.; Chen, N.-F.; Wen, Z.-H.; Fang, L.-S.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Natural product chemistry of gorgonian corals of genus Junceella—Part III. Mar. Drugs 2018, 16, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.-H.; Zhang, S.; Wen, Y.-M.; Xiao, Z.-H.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella fragilis. Helv. Chim. Acta 2005, 88, 2349–2354. [Google Scholar] [CrossRef]
- Lin, Y.; Long, K. Studies of the chemical constituents of the Chinese gorgonia (IV)-Junceella, a new chlorine-containing diterpenoid from Junceella squamata. Acta Sci. Nat. Univ. Sunyatseni 1983, (2), 46–51. [Google Scholar]
- Yao, J.; Qian, J.; Fan, H.; Shih, K.; Huang, S.; Lin, Y.; Long, K. The structure of crystal and molecule of junceellin. Acta Sci. Nat. Univ. Sunyatseni 1984, (1), 83–87. [Google Scholar]
- Shin, J.; Park, M.; Fenical, W. The junceellolides, new anti-inflammatory diterpenoids of the briarane class from the Chinese gorgonian Junceella fragilis. Tetrahedron 1989, 45, 1633–1638. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kulatheeswaran, R.; Ward, R.S. Briarane diterpenes from the Indian Ocean gorgonian Gornonella umbraculum. J. Nat. Prod. 1998, 61, 1120–1122. [Google Scholar] [CrossRef]
- García, M.; Rodríguez, J.; Jiménez, C. Absolute structures of new briarane diterpenoids from Junceella fragilis. J. Nat. Prod. 1999, 62, 257–260. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Wu, S.-L.; Li, J.-J.; Chen, M.-C.; Cheng, Y.-M.; Wang, G.-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2003, 51, 1429–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, P.-J.; Fan, T.-Y.; Chen, M.-C.; Fang, L.-S.; Lin, M.-R.; Chang, P.-C. Junceellin and praelolide, two briaranes from the gorgonian corals Junceella fragilis and Junceella juncea (Ellisellidae). Biochem. Syst. Ecol. 2004, 32, 111–113. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Shen, Y.-C.; Lin, Y.-S.; Hwang, T.-L.; Kuo, Y.-H.; Khalil, A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chen, W.-F.; Lee, G.-H.; Wen, Z.-H.; Fang, L.-S.; Kuo, Y.-H.; Lee, C.-Y.; Sung, P.-J. Fragilides M–O, new triacetoxybriaranes from the gorgonian coral Junceella fragilis (Ellisellidae). Heterocycles 2019, 98, 984–993. [Google Scholar]
- Sheu, J.-H.; Chen, Y.-P.; Hwang, T.-L.; Chiang, M.Y.; Fang, L.-S.; Sung, P.-J. Junceellolides J–L, 11,20- epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 2006, 69, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Yamamoto, Y.; Otsuka, M.; Tanaka, J.; Ichiba, T.; Marriott, G.; Rachmat, R.; Higa, T. Briarane diterpenes from two species of octocorals, Ellisella sp. and Pteroeides sp. J. Nat. Prod. 2004, 67, 1368–1373. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chiang, M.Y.; Tsai, W.-T.; Su, J.-H.; Su, Y.-M.; Wu, Y.-C. Chlorinated briarane-type diterpenoids from the gorgonian coral Ellisella robusta (Ellisellidae). Tetrahedron 2007, 63, 12860–12865. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Liaw, C.-C.; Fazary, A.E.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from the gorgonian coral Junceella juncea. Mar. Drugs 2012, 10, 1321–1330. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Lin, Y.-C.; Lin, Y.-S.; Chen, C.-H.; Hwang, T.-L.; Shen, Y.-C. Four new briarane diterpenoids from Taiwanese gorgonian Junceella fragilis. Mar. Drugs 2013, 11, 2042–2053. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Jean, Y.-H.; Chen, W.-F.; Sung, C.-S.; Duh, C.-Y.; Huang, S.-Y.; Lin, C.-S.; Tai, M.-H.; Tzeng, S.-F.; Wen, Z.-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Lin, Y.-Y.; Jean, Y.-H.; Lu, Y.; Chen, W.-F.; Yang, S.-N.; Wang, H.-M.D.; Jang, I.-Y.; Chen, I.-M.; Su, J.-H.; et al. Anti-inflammatory and analgesic effects of the marine- derived compound comaparvin isolated from the crinoid Comanthus bennetti. Molecules 2014, 19, 14667–14686. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.; Figueiredo, C.A.; Brito, C.; Stavroullakis, A.; Prakki, A.; da Silva Velozo, E.; Nogueira-Filho, G. Effect of Allium cepa L. on lipopolysaccharide-stimulated osteoclast precursor cell viability, count, and morphology using 4′,6-diamidino-2-phenylindole-staining. Int. J. Cell Biol. 2014, 2014, 535789. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Li, X.; Yin, F.; van Ofwegen, L.; Lin, W. Halogenated briarane diterpenes with acetyl migration from the gorgonian coral Junceella fragilis. Chem. Biodiver. 2017, 14, e1700053. [Google Scholar] [CrossRef]
- Cheng, W.; Ji, M.; Li, X.; Ren, J.; Yin, F.; van Ofwegen, L.; Yu, S.; Chen, X.; Lin, W. Fragilolides A–Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-M.; Fan, T.-Y.; Sung, P.-J. 11,20-Epoxybriaranes from the gorgonian coral Ellisella robusta (Ellisellidae). Nat. Prod. Res. 2007, 21, 1085–1090. [Google Scholar] [CrossRef]

 ) correlations, selective heteronuclear multiple bond correlation (HMBC) experiment (
) correlations, selective heteronuclear multiple bond correlation (HMBC) experiment (  ), and protons with key nuclear Overhauser effect spectroscopy (NOESY) (
), and protons with key nuclear Overhauser effect spectroscopy (NOESY) (  ) correlations of 1.
) correlations of 1.
 ) correlations, selective heteronuclear multiple bond correlation (HMBC) experiment (
) correlations, selective heteronuclear multiple bond correlation (HMBC) experiment (  ), and protons with key nuclear Overhauser effect spectroscopy (NOESY) (
), and protons with key nuclear Overhauser effect spectroscopy (NOESY) (  ) correlations of 1.
) correlations of 1.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and protons with key NOESY (
), and protons with key NOESY (  ) correlations of 2.
) correlations of 2.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and protons with key NOESY (
), and protons with key NOESY (  ) correlations of 2.
) correlations of 2.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and protons with key NOESY (
), and protons with key NOESY (  ) correlations of 3.
) correlations of 3.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and protons with key NOESY (
), and protons with key NOESY (  ) correlations of 3.
) correlations of 3.

| Position | 1 a | 2 a | 3 b |
|---|---|---|---|
| 1 | 47.0, C c | 46.1, C | 49.1, C |
| 2 | 72.2, CH | 72.1, CH | 75.4, CH |
| 3 | 37.5, CH2 | 37.6, CH2 | 136.4, CH |
| 4 | 70.6, CH | 73.4, CH | 128.2, CH |
| 5 | 142.0, C | 144.1, C | 135.9, C |
| 6 | 120.6, CH | 58.0, CH | 128.8, CH |
| 7 | 76.5, CH | 79.0, CH | 80.0, CH |
| 8 | 80.4, C | 81.9, C | 80.9, C |
| 9 | 67.4, CH | 68.9, CH | 67.1, CH |
| 10 | 39.9, CH | 39.8, CH | 39.5, CH |
| 11 | 62.5, C | 62.6, C | 60.8, C |
| 12 | 23.6, CH2 | 23.5, CH2 | 29.4, CH2 |
| 13 | 24.2, CH2 | 24.0, CH2 | 25.5, CH2 |
| 14 | 73.2, CH | 73.5, CH | 78.9, CH |
| 15 | 14.6, CH3 | 15.4, CH3 | 16.0, CH3 |
| 16 | 21.4, CH3 | 121.0, CH2 | 48.0, CH2 |
| 17 | 42.4, CH | 43.8, CH | 45.2, CH |
| 18 | 6.6, CH3 | 7.1, CH3 | 6.9, CH3 |
| 19 | 175.9, C | 175.2, C | 174.8, C |
| 20 | 59.1, CH2 | 58.2, CH2 | 50.4, CH2 |
| Acetate methyls | 21.7, CH3 | 21.9, CH3 | 21.7, CH3 |
| 21.2, CH3 | 21.0, CH3 | 21.4, CH3 | |
| 20.9, CH3 | 21.0, CH3 | 21.3, CH3 | |
| 20.8, CH3 | |||
| Acetate carbonyls | 170.1, C | 171.0, C | 170.4, C |
| 169.8, C | 170.1, C | 169.7, C | |
| 169.4, C | 169.7, C | 169.3, C | |
| 169.0, C |
| Position | 1 a | 2 a | 3 b |
|---|---|---|---|
| 2 | 5.03 br s | 4.96 dd (2.8, 2.8) | 5.63 d (6.0) |
| 3α/β | 2.04 m; 2.62 ddd (18.4, 4.0, 2.4) | 1.75 m; 2.38 m | 6.04 dd (17.4, 6.0) |
| 4 | 5.91 br s | 4.85 m | 6.42 d (17.4) |
| 6 | 5.67 dq (8.8, 1.6) | 5.49 br s | 5.66 d (3.6) |
| 7 | 5.04 d (8.8) | 5.15 d (2.4) | 5.62 d (3.6) |
| 9 | 5.65 d (5.6) | 5.52 d (6.4) | 4.77 d (6.6) |
| 10 | 2.36 dd (5.6, 1.6) | 2.31 d (6.4) | 3.32 d (6.6) |
| 12α/β | 1.13 m; 2.30 m | 1.15 m; 2.30 m | 1.29 m; 2.18 m |
| 13α/β | 2.11 m; 1.81 m | 2.14 m; 1.78 m | 2.08 m; 1.85 dddd (12.0, 12.0, 3.6, 1.8) |
| 14 | 4.71 d (4.8) | 4.86 d (4.4) | 4.94 dd (3.6, 3.0) |
| 15 | 1.09 s | 1.13 s | 0.87 s |
| 16a/b | 2.00 d (1.6) | 5.74 s; 5.97 s | 4.13 d (12.0); 4.19 d (12.0) |
| 17 | 2.33 q (7.6) | 2.29 q (7.6) | 2.36 q (7.2) |
| 18 | 1.15 d (7.6) | 1.22 d (7.6) | 1.18 d (7.2) |
| 20a/b | 2.85 d (4.4); 2.98 dd (4.4, 1.6) | 2.83 d (4.0); 2.85 br d (4.0) | 2.76 d (2.4); 3.40 dd (2.4, 2.4) |
| OH-8 | 4.85 br s | 4.63 br s | 3.13 d (1.2) |
| Acetate methyls | 2.24 s | 2.23 s | 2.27 s |
| 2.01 s | 2.03 s | 2.25 s | |
| 1.99 s | 1.99 s | 2.11 s | |
| 1.98 s |
| iNOS | COX-2 | β-Actin | |
|---|---|---|---|
| Compound | Expression (% of LPS) | ||
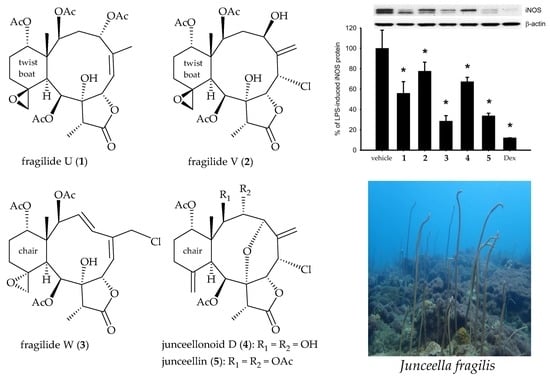
| Lipopolysaccharides | 100.01 ± 17.81 | 100.00 ± 3.04 | 100.00 ± 6.05 |
| 1 | 55.88 ± 11.42 | 64.73 ± 4.07 | 90.05 ± 7.11 |
| 2 | 77.58 ± 8.82 | 66.23 ± 6.59 | 99.29 ± 5.24 |
| 3 | 28.55 ± 5.35 | 86.46 ± 8.46 | 101.69 ± 6.46 |
| 4 | 67.25 ± 4.27 | 130.88 ± 13.94 | 103.20 ± 2.56 |
| 5 | 33.72 ± 2.57 | 64.15 ± 3.03 | 84.52 ± 7.78 |
| Dexamethasone | 12.11 ± 0.03 | 2.11 ± 0.44 | 123.86 ± 2.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.-P.; Yuan, C.-H.; Jhu, Y.-M.; Peng, B.-R.; Wen, Z.-H.; Wu, Y.-J.; Wu, T.-Y.; Liu, H.-W.; Sung, P.-J. Fragilides U–W: New 11,20-Epoxybriaranes from the Sea Whip Gorgonian Coral Junceella fragilis. Mar. Drugs 2019, 17, 706. https://doi.org/10.3390/md17120706
Su T-P, Yuan C-H, Jhu Y-M, Peng B-R, Wen Z-H, Wu Y-J, Wu T-Y, Liu H-W, Sung P-J. Fragilides U–W: New 11,20-Epoxybriaranes from the Sea Whip Gorgonian Coral Junceella fragilis. Marine Drugs. 2019; 17(12):706. https://doi.org/10.3390/md17120706
Chicago/Turabian StyleSu, Tung-Pin, Chien-Han Yuan, Yi-Ming Jhu, Bo-Rong Peng, Zhi-Hong Wen, Yu-Jen Wu, Tung-Ying Wu, Hong-Wen Liu, and Ping-Jyun Sung. 2019. "Fragilides U–W: New 11,20-Epoxybriaranes from the Sea Whip Gorgonian Coral Junceella fragilis" Marine Drugs 17, no. 12: 706. https://doi.org/10.3390/md17120706
APA StyleSu, T. -P., Yuan, C. -H., Jhu, Y. -M., Peng, B. -R., Wen, Z. -H., Wu, Y. -J., Wu, T. -Y., Liu, H. -W., & Sung, P. -J. (2019). Fragilides U–W: New 11,20-Epoxybriaranes from the Sea Whip Gorgonian Coral Junceella fragilis. Marine Drugs, 17(12), 706. https://doi.org/10.3390/md17120706