New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata
Abstract
:1. Introduction
2. Results and Discussion
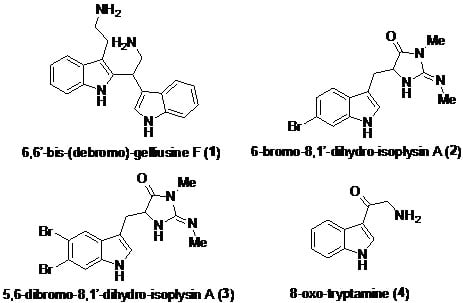
2.1. Chemistry
2.2. Microfouling Activity
2.3. Antiplasmodial Activity
3. Materials and Methods
3.1. General Experiment Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. In Vitro Antiplasmodial Assays
3.5. In Vitro Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bialonska, D.; Zjawiony, J.K. Aplysinopsins—Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance. Mar. Drugs 2009, 7, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Roll, D.M.; Ireland, C.M.; Lu, H.S.M.; Clardy, J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J. Org. Chem. 1988, 53, 3276–3278. [Google Scholar] [CrossRef]
- Segraves, N.L.; Robinson, S.J.; Garcia, D.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Comparison of Fascaplysin and Related Alkaloids: A Study of Structures, Cytotoxicities, and Sources. J. Nat. Prod. 2004, 67, 783–792. [Google Scholar] [CrossRef]
- Jimenez, C.; Quinoa, E.; Adamczeski, M.; Hunter, L.M.; Crews, P. Novel sponge-derived amino acids. 12. Tryptophan-derived pigments and accompanying sesterterpenes from Fascaplysinopsis reticulata. J. Org. Chem. 1991, 56, 3403–3410. [Google Scholar] [CrossRef]
- Kirsch, G.; König, G.M.; Wright, A.D.; Kaminsky, R. A New Bioactive Sesterterpene and Antiplasmodial Alkaloids from the Marine Sponge Hyrtios cf. erecta. J. Nat. Prod. 2000, 63, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Quiñoá, E.; Crews, P. Novel marine sponge alkaloids 3. β-carbolinium salts from Fascaplysinopsis reticulata. Tetrahedron Lett. 1991, 32, 1843–1846. [Google Scholar] [CrossRef]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla—Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003, 44, 3471–3475. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Luo, X.; de Voogd, N.J.; Li, P.; Li, G. (+)- and (−)-Spiroreticulatine, A Pair of Unusual Spiro Bisheterocyclic Quinoline-imidazole Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Org. Lett. 2015, 17, 3458–3461. [Google Scholar] [CrossRef]
- Bodendorf, K.; Walk, A. Darstellung und Reduktion von Indolyl-(3)-aminomethyl-ketonen. Arch. Pharm. 1961, 294, 484–487. [Google Scholar] [CrossRef]
- Seetham Naidu, P.; Bhuyan, P.J. Synthesis of some analogues of (±)gelliusine F, (±)gelliusine E, and total synthesis of 2,2-di(6′-bromo-3′-indolyl)ethylamine. Tetrahedron Lett. 2012, 53, 426–428. [Google Scholar] [CrossRef]
- Bifulco, G.; Bruno, I.; Riccio, R.; Lavayre, J.; Bourdy, G. Further Brominated Bis- and Tris-Indole Alkaloids from the Deep-Water New Caledonian Marine Sponge Orina sp. J. Nat. Prod. 1995, 58, 1254–1260. [Google Scholar] [CrossRef]
- Benbouzid-Rollet, N.D.; Conte, M.; Guezennec, J.; Prieur, D. Monitoring of a Vibrio natriegens and Desulfovibrio vulgaris marine aerobic biofilm on a stainless steel surface in a laboratory tubular flow system. J. Appl. Bacteriol. 1991, 71, 244–251. [Google Scholar] [CrossRef]
- Cheng, G.; Li, G.; Xue, H.; Chen, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.K.; Liu, P.C.; Chuang, W.H. Pathogenesis of gastroenteritis caused by Vibrio carchariae in cultured marine fish. Mar. Biotechnol. 2002, 4, 267–277. [Google Scholar] [CrossRef]
- Nicolas, J.L.; Basuyaux, O.; Mazurie, J.; Thebault, A. Vibrio carchariae, a pathogen of the abalone Haliotis tuberculata. Dis. Aquat. Org. 2002, 50, 35–43. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Thompson, F.L.; Gomez-Gil, B.; Swings, J. Phenotypic diversity amongst Vibrio isolates from marine aquaculture systems. Aquaculture 2003, 219, 9–20. [Google Scholar] [CrossRef]
- Hu, J.-F.; Schetz, J.A.; Kelly, M.; Peng, J.-N.; Ang, K.K.H.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P.; et al. New Antiinfective and Human 5-HT2 Receptor Binding Natural and Semisynthetic Compounds from the Jamaican Sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef]
- Frédérich, M.; Jacquier, M.J.; Thepenier, P.; De Mol, P.; Tits, M.; Philippe, G.; Delaude, C.; Angenot, L.; Zeches-Hanrot, M.J. Antiplasmodial activity of alkaloids from various Strychnos species. J. Nat. Prod. 2002, 65, 1381–1386. [Google Scholar] [CrossRef]
- Jonville, M.C.; Dive, G.; Angenot, L.; Bero, J.; Tits, M.; Ollivier, E.; Frédérich, M. Dimeric bisindole alkaloids from the stem bark of Strychnos nux-vomica L. Phytochemistry 2013, 87, 156–163. [Google Scholar] [CrossRef]
- Thabard, M.; Gros, O.; Hellio, C.; Maréchal, J.P. Sargassum polyceratium (Phaeophyceae, Fucaceae) surface molecule activity towards fouling organisms and embryonic development of benthic species. Bot. Mar. 2011, 54, 147–157. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Pile, C.; Pavia, H.; Hellio, C.; Svenson, J. Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 2015, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]


| Position | δC, Type | δH (J in Hz) | COSY (1H-1H) | HMBC (1H-13C) |
|---|---|---|---|---|
| 2 | 124.0, C | - | - | - |
| 3 | 113.8, C | - | - | - |
| 3a | 127.5, C | - | - | - |
| 4 | 118.9, CH | 7.54, d (7.8) | 5 | 6, 7a |
| 5 | 112.6, CH | 7.38, m | 4, 6 | 3a, 7 |
| 6 | 123.1, CH | 7.12, m | 5, 7 | 4, 7a |
| 7 | 120.7, CH | 7.06, m | 6 | 3a, 5 |
| 7a | 135.3, C | - | - | - |
| 8 | 23.7, CH2 | 3.23, m | 9 | 2, 3, 3a, 9 |
| 9 | 41.4, CH2 | 3.00, m | 8 | 3, 8 |
| 2′ | 124.0, CH | 7.27, s | - | 3′, 3a’, 7a’ |
| 3′ | 113.7, C | - | - | - |
| 3a’ | 129.2, C | - | - | - |
| 4′ | 119.3, CH | 7.58, d (7.8) | 5′ | 6′, 7a’ |
| 5′ | 112.9, CH | 7.41, m | 4′, 6′ | 3a’, 7′ |
| 6′ | 123.3, CH | 7.14, m | 5′, 7′ | 4′, 7a’ |
| 7′ | 120.6, CH | 7.06, m | 6′ | 3a’, 5′ |
| 7a’ | 138.0, C | - | - | - |
| 8′ | 34.3, CH | 5.10, t (8.6) | 9′ | 2′, 3′, 3a’, 9′ |
| 9′ | 44.3, CH2 | 3.83–3.69 (m) | 8′ | 3′, 8′ |
| Position | δC, Type | δH (J in Hz) | COSY (1H-1H) | HMBC (1H-13C) |
|---|---|---|---|---|
| 2 | 126.4, CH | 7.11, s | - | 3, 3a, 7a, 8 |
| 3 | 109.0, C | - | - | - |
| 3a | 127.6, C | - | - | - |
| 4 | 121.1, CH | 7.51, d (8.6) | 5 | 6, 7a |
| 5 | 123.3, CH | 7.14, dd (8.6, 1.8) | 4 | 3a, 7 |
| 6 | 116.6, C | - | - | - |
| 7 | 115.5, CH | 7.50, d (1.8) | - | 3a, 5 |
| 7a | 138.1, C | - | - | - |
| 8 | 28.1, CH2 | 3.35, m | 1′ | - |
| 1′ | 61.8, CH | 4.62, t (4.9) | 8 | 5′, 8 |
| 3′ | 159.2, C | - | - | - |
| 5′ | 174.9, C | - | - | - |
| 6′ | 25.9, CH3 | 2.90, s | - | 3′ |
| 7′ | 29.3, CH3 | 2.86, s | -’ | 3′, 5′ |
| Position | δH (J in Hz) | δC, Type | ||
|---|---|---|---|---|
| 6-Bromo-8,1′-dihydro-isoplysin A (2) | 5,6-Dibromo-8,1′-dihydro-isoplysin A (3) | 6-Bromo-8,1′-dihydro-isoplysin A (2) | 5,6-Dibromo-8,1′-dihydro-isoplysin A (3) | |
| 2 | 7.11, s | 7.16, s | 126.4, CH | 126.6, CH |
| 3 | - | - | 109.0, C | 109.0, C |
| 3a | - | - | 127.6, C | 129.8, C |
| 4 | 7.51, d (8.6) | 7.96, s | 121.1, CH | 123.6, CH |
| 5 | 7.14, dd (8.6, 1.8) | - | 123.3, CH | 116.9, C |
| 6 | - | - | 116.6, C | 115.9, C |
| 7 | 7.50, d (1.8) | 7.69, s | 115.5, CH | 117.4, CH |
| 7a | - | - | 138.1, C | 137.5, C |
| 8 | 3.35, m | 3.73, m | 28.1, CH2 | 28.2, CH2 |
| 1′ | 4.62, t (4.9) | 4.60, t (5.3) | 61.8, CH | 61.4, CH |
| 3′ | - | - | 159.2, C | 157.9, C |
| 5′ | - | - | 174.9, C | 175.8, C |
| 6′ | 2.90, s | 2.86, s | 25.9, CH3 | 25.4, CH3 |
| 7′ | 2.86, s | 2.94, s | 29.3, CH3 | 28.9, CH3 |
| Compounds | Shewanellia putrefaciens MIC, µg/mL | Roseobacter littoralis MIC, µg/mL | Vibrio carchariae MIC, µg/mL | Vibrio natrigens MIC, µg/mL | Vibrio proteolyticus MIC, µg/mL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | G | A | G | A | G | A | G | A | G | |
| 6,6′-bis-(debromo)-gelliusine F (1) | - | - | - | - | - | - | - | - | - | - |
| 6-bromo-8,1′-dihydro-isoplysin A (2) | - | 100 | - | - | 100 | - | 100 | 0.01 | - | - |
| 5,6-dibromo-8,1′-dihydro-isoplysin A (3) | - | - | - | - | - | - | - | 1 | - | - |
| 8-oxo-tryptamine (4) | - | - | - | - | - | - | - | - | - | - |
| tryptamine (5) | - | - | - | - | - | 1 | - | - | - | - |
| (E) and (Z)-6-bromo-2′-demethyl-3′-N-methylaplysinopsine (6 + 7) | - | - | - | - | - | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, P.-E.; Pichon, E.; Moriou, C.; Clerc, P.; Trépos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata. Mar. Drugs 2019, 17, 167. https://doi.org/10.3390/md17030167
Campos P-E, Pichon E, Moriou C, Clerc P, Trépos R, Frederich M, De Voogd N, Hellio C, Gauvin-Bialecki A, Al-Mourabit A. New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata. Marine Drugs. 2019; 17(3):167. https://doi.org/10.3390/md17030167
Chicago/Turabian StyleCampos, Pierre-Eric, Emmanuel Pichon, Céline Moriou, Patricia Clerc, Rozenn Trépos, Michel Frederich, Nicole De Voogd, Claire Hellio, Anne Gauvin-Bialecki, and Ali Al-Mourabit. 2019. "New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata" Marine Drugs 17, no. 3: 167. https://doi.org/10.3390/md17030167
APA StyleCampos, P. -E., Pichon, E., Moriou, C., Clerc, P., Trépos, R., Frederich, M., De Voogd, N., Hellio, C., Gauvin-Bialecki, A., & Al-Mourabit, A. (2019). New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata. Marine Drugs, 17(3), 167. https://doi.org/10.3390/md17030167