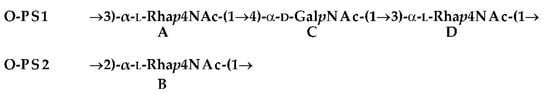A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6
Abstract
:- O-PS1
- →3)-α-l-Rhap4NAc-(1→4)-α-d-GalpNAc-(1→3)-α-l-Rhap4NAc-(1→
- O-PS2
- →2)-α-l-Rhap4NAc-(1→
1. Introduction
2. Results
2.1. Bacterial Cultivation, Isolation of LPS, and SDS-PAGE Study
2.2. Chemical and Mass Spectrometry Analyses of LPS
2.3. Structural Studies of O-polysaccharide (O-PS)
| O-PS1 | →3)-α-l-Rhap4NAc-(1→4)-α-d-GalpNAc-(1→3)-α-l-Rhap4NAc-(1→ | ||
| A | C | D | |
| O-PS2 | →2)-α-l-Rhap4NAc-(1→ | ||
| B | |||
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Cultivation Conditions, and Isolation of LPS
4.2. Degradation of LPS and Isolation of O-polysaccharide
4.3. Chemical Analyses
4.4. NMR Spectroscopy
4.5. MALDI-TOF Mass Spectrometry (MS)
4.6. SDS-PAGE
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janda, J.M.; Duffy, P.S. Mesophilic aeromonads in human diseases: Current taxonomy, laboratory infection and infectious diseases spectrum. Rev. Infect. Dis. 1988, 10, 980–997. [Google Scholar] [CrossRef]
- Janda, J.M. Recent advances in the study of the taxonomy, pathogenicity and infectious syndromes with the genus Aeromonas. Clin. Microbiol. Rev. 1991, 4, 397–410. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, S.A.; Khan, A.A.; Sung, K.; Tran, Q.; Kerdahi, K.; Steele, R. Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol. 2010, 27, 327–331. [Google Scholar] [CrossRef]
- Araujo, R.M.; Arribas, R.M.; Pares, R. Distribution of Aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 1991, 71, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Holmberg, S.D.; Schell, W.L.; Fanning, G.R.; Wachsmuth, I.K.; Blake, P.A.; Brenner, D.J.; Farmer, J.J. Aeromonas intestinal infections in the United States. Ann. Int. Med. 1986, 105, 683–689. [Google Scholar] [CrossRef]
- Ali, A.; Carnahan, A.M.; Altwegg, M.; Luthy-Hottenstein, J.; Joseph, S.W. Aeromonas bestiarum sp. nov. (formerly genomospecies DNA group 2 A. hydrophila), a new species isolated from non human sources. Med. Microbiol. Lett. 1996, 5, 156–165. [Google Scholar]
- Kahajanchi, B.K.; Fadl, A.A.; Borchardt, M.A.; Berg, R.L.; Horneman, A.J.; Stemper, M.E.; Joseph, S.W.; Moyer, N.P.; Sha, J.; Chopra, A.K. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: Suggestive evidence of water-to-human transmission. Appl. Environ. Microbiol. 2010, 76, 2313–2325. [Google Scholar] [CrossRef]
- Figueras, M.J. Clinical relevance of Aeromonas sM503. Rev. Clin. Microbiol. 2005, 16, 145–153. [Google Scholar] [CrossRef]
- Tomas, J.M. The Main Aeromonas Pathogenic Factors. ISRN Microbiology. 2012, 22. [Google Scholar] [CrossRef]
- Dooley, J.S.G.; Lallier, R.; Shaw, D.H.; Trust, T.J. Electrophoretic and immunochemical analyses of the lipopolysaccharides from various strains of Aeromonas hydrophila. J. Bacteriol. 1985, 164, 263–269. [Google Scholar] [PubMed]
- Merino, S.; Rubires, X.; Aguillar, A.; Guillot, J.F.; Tomas, J.M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Aeromonas hydrophila serogroup O:34. Microb. Pathog. 1996, 20, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Merino, S.; Rubires, X.; Tomas, J. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37 °C. Infect. Immun. 1997, 65, 1245–1250. [Google Scholar]
- Rabaan, A.A.; Gryllos, I.; Tomas, J.M.; Shaw, J.G. Motility and polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect. Immun. 2001, 69, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Garduño, R.A.; Moore, A.R.; Oliver, G.; Lizama, A.L.; Garduño, E.; Kay, W.W. Host cell invasion and intracellular resistance by Aeromonas salmonicida: Role of the S-layer. J. Clin. Microbiol. 2000, 46, 660–668. [Google Scholar]
- Asha, A.; Nayak, D.K.; Shankar, K.M.; Mohan, C.V. Antigen expression in biofilm cells of Aeromonas hydrophila employed in oral vaccination of fish. Fish Shellfish Immun. 2004, 16, 429–436. [Google Scholar] [CrossRef]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Nazarenko, E.L.; Crawford, R.J.; Iwanowa, E.P. The structural diversity of carbohydrate antigens of selected Gram-negative marine bacteria. Mar. Drugs 2011, 9, 1914–1954. [Google Scholar] [CrossRef] [PubMed]
- Sakazaki, R.; Shimada, T. O-Serogrouping for mesophilic Aeromonas strains. Jpn. J. Med. Sci. Biol. 1984, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Gross, R.J.; Cheasty, T.; Rowe, B. Extended serogrouping scheme for motile, mesophilic Aeromonas species. J. Clin. Microbiol. 1990, 28, 980–984. [Google Scholar]
- Esteve, C.; Alcaide, E.; Canals, R.; Merino, S.; Blasco, D.; Figueras, M.J.; Tomas, J.M. Pathogenic Aeromonas hydrophila serogroup O:14 and O:81 strains with S-layer. Appl. Environ. Microbiol. 2004, 70, 5898–5904. [Google Scholar] [CrossRef]
- Kozinska, A.; Pekala, A. Serotyping of Aeromonas species isolated from Polish fish farms in relation to species and virulence phenotype of the bacteria. Bull. Vet. Inst. Pulawy 2010, 54, 315–320. [Google Scholar]
- Show, D.H.; Squires, M.J. O-antigen structure in a virulent strain Aeromonas hydrophila. FEMS Microbiol. Lett. 1984, 24, 277–281. [Google Scholar] [CrossRef]
- Merino, S.; Canals, R.; Knirel, Y.A.; Tomas, J.M. Molecular and chemical analysis of the lipopolysaccharide from Aeromonas hydrophila strain AH-1 (Serotype O11). Mar. Drugs 2015, 13, 2233–2249. [Google Scholar] [CrossRef]
- Pieretti, G.; Carillo, S.; Lanzetta, R.; Parrilli, M.; Merino, S.; Tomas, J.M.; Corsaro, M.M. Structural determination of the O-specific polysaccharide from Aeromonas hydrophila strain A19 (serogroup O:14) with S-layer. Carbohydr. Res. 2011, 346, 2519–2522. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Shashkov, A.S.; Senchenkova, S.N.; Merino, S.; Tomas, J.M. Structure of the O-specific polysaccharide of Aeromonas hydrophila O:34; a case of random O-acetylation of 6-deoxy-L-talose. Carbohydr. Res. 2002, 337, 1381–1386. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Duda, K.A.; Schwudke, D.; Pekala, A.; Kozinska, A.; Holst, O. Structural studies of the lipopolysaccharide from the fish pathogen, Aeromonas veronii strain Bs19, serotype O16. Mar. Drugs 2014, 12, 1298–1316. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Lindner, B.; Komaniecka, I.; Kozinska, A.; Pekala, A.; Choma, A.; Holst, O. Structural and immunochemical studies of the lipopolysaccharide from the fish pathogen, Aeromonas bestiarum strain K296, serotype O18. Mar. Drugs 2013, 11, 1235–1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Vinogradov, E.; Larocque, S.; Harrison, B.A.; Li, J.; Altman, E. Structural and serological characterization of the O-chain polysaccharide of Aeromonas salmonicida strains A449, 80204 and 80204–1. Carbohydr. Res. 2005, 340, 693–700. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Dacanay, A.; Harrison, B.A.; Fast, M.; Colquhoun, D.J.; Lund, V.; Brown, L.L.; Li, J.; Altman, E. Carbohydrate analysis and serological classification of typical and atypical isolates of Aeromonas salmonicida: a rationale for the lipopolysaccharide-based classification of A. salmonicida. Fish Shellfish Immun. 2007, 23, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Li, J.; Altman, E. Structural characterization of the O-chain polysaccharide of Aeromonas caviae ATCC 15468 lipopolysaccharide. Carbohydr. Res. 2008, 343, 483–488. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Pietras, H.; Duda, K.A.; Kozińska, A.; Pękala, A.; Holst, O. Structure of the O-specific polysaccharide from the lipopolysaccharide of Aeromonas sobria strain Pt312. Carbohydr. Res. 2015, 403, 142–148. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Kozinska, A.; Russa, R.; Holst, O. The structure of the O-specific polysaccharide from the lipopolysaccharide of Aeromonas bestiarum strain 207. Carbohydr. Res. 2010, 345, 680–684. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Guz, L.; Lindner, B.; Pietras, H.; Russa, R.; Holst, O. Structural characterization of the O-specific polysaccharide from the lipopolysaccharide of fish pathogen Aeromonas bestiarum strain P1S. Carbohydr. Res. 2011, 346, 815–821. [Google Scholar] [CrossRef]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide. Extraction with phenol-water and further applications of the procedure. Meth. Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Knirel, Y.A.; Vinogradov, E.; Jimenez, N.; Merino, S.; Tomas, J.M. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr. Res. 2004, 339, 787–793. [Google Scholar] [CrossRef]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Nicolaus, B.; Gambacorta, A.; Lindner, B.; Holst, O. Structural characterization of the core region of the lipopolysaccharide from the haloalkaliphilic Halomonas pantelleriensis: identification of the biological O-antigen repeating unit. Eur. J. Org. Chem. 2008, 721–728. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systamatic nomenclature for carbohydrate fragmentations in FAB MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Jimenez, N.; Lacasta, A.; Vilches, S.; Reyes, M.; Vazquez, J.; Aquillini, E.; Merino, S.; Regue, M.; Tomas, J.M. Genetics and proteomics of Aeromonas salmonicida lipopolysaccharide core biosynthesis. J. Bacteriol. 2009, 191, 2228–2236. [Google Scholar] [CrossRef]
- Lipiński, T.; Zatonsky, G.V.; Kocharova, N.A.; Jaquinod, M.; Forest, E.; Shashkov, A.S.; Gamian, A.; Knirel, Y.A. Structures of two O-chain polysaccharides of Citrobacter gillenii O9a,9b lipopolysaccharide. A new homopolymer of 4-amino-4,6-dideoxy-D-mannose (perosamine). Eur. J. Biochem. 2002, 269, 93–99. [Google Scholar] [CrossRef]
- Leontein, K.; Lindberg, B.; Lönngren, J. Assignment of absolute configuration of sugars by GLC of their acetylated glycosides formed from chiral alcohols. Carbohydr. Res. 1978, 62, 359–362. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Shashkov, A.S.; Knirel, Y.A.; Vinogradov, E.V.; Kochetkov, N.K. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydr. Res. 1988, 175, 59–75. [Google Scholar] [CrossRef]
- Ovchinnikova, O.G.; Kocharova, N.A.; Katzenellenbogen, E.; Zatonsky, G.V.; Shashkov, A.S.; Knirel, Y.A.; Lipiński, T.; Gamian, A. Structures of two O-polysaccharides of the lipopolysaccharide of Citrobacter youngae PCM 1538 (serogroup O9). Carbohydr. Res. 2004, 339, 881–884. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Widmalm, G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-NMR data. Carbohydr. Res. 1989, 188, 169–191. [Google Scholar] [CrossRef]
- Shashkov, A.S.; Lipkind, G.M.; Knirel, Y.A.; Kochetkov, N.K. Stereochemical factors determining the effects of glycosylation on the 13C NMR shifts in carbohydrates. Magn. Reson. Chem. 1988, 26, 735–747. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Kaszowska, M.; Wojcik, M.; Sudnienko, J.; Lugowski, C.; Lukasiewicz, J. Structure-activity relationship of Plesiomonas shigelloides lipid A to the production of TNF-α, IL-1β, and IL-6 by human and murine macrophages. Front. Immunol. 2017, 8, 1741. [Google Scholar] [CrossRef]
- Karaś, M.A.; Turska-Szewczuk, A.; Janczarek, M.; Szuster-Ciesielska, A. Glycoconjugates of Gram-negative bacteria and parasitic protozoa – are they similar in orchestrating the innate immune response? Innate Immun. 2019, 25, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Arteaga Garibay, R.I.; Aguilera-Arreola, M.G.; Navarro Ocana, A.; Giono Cerezo, S.; Sanchez Mendoza, M.; Molina Lopez, J.; Eslava Campos, C.; Cravioto, A.; Castro-Escarpulli, G. Serogroups, K1 antigen, and antimicrobial resistance patterns of Aeromonas spp. strains isolated from different sources in Mexico. Mem. Inst. Oswaldo Cruz 2006, 101, 157–161. [Google Scholar] [CrossRef]
- Bundle, D.R.; Cherwonogrodzky, J.W.; Perry, M.B. The structure of the lipopolysaccharide O-chain (M antigen) and polysaccharide B produced by Brucella melitensis 16M. FEBS Lett. 1987, 216, 261–264. [Google Scholar] [CrossRef]
- Kubler-Kielb, J.; Vinogradov, E. Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res. 2013, 378, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Kocharova, N.A.; Bystrova, O.V.; Katzenellenbogen, E.; Gamian, A. Structures and serology of the O-specific polysaccharides of bacteria of the genus Citrobacter. Arch. Immunol. Ther. Exp. 2002, 50, 379–391. [Google Scholar]
- Caroff, M.; Bundle, D.R.; Perry, M.B.; Cherwonogrodzky, J.W.; Dunkan, J.R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119–3. Infect. Immun. 1984, 46, 384–388. [Google Scholar]
- Caroff, M.; Bundle, D.R.; Perry, M.B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur. J. Biochem. 1984, 139, 195–200. [Google Scholar] [CrossRef]
- Kenne, L.; Lindberg, B.; Unger, P.; Gustafsson, B.; Holme, T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr. Res. 1982, 100, 341–349. [Google Scholar] [CrossRef]
- Haishima, Y.; Kondo, S.; Hisatsune, K. The occurrence of α(1→2) linked N-acetylperosamine-homopolymer in lipopolysaccharides of non-O1 Vibrio cholerae possessing an antigenic factor in common with O1 V. cholerae. Microbiol. Immunol. 1990, 34, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.B.; MacLean, L.; Griffith, D.W. Structure of the O-chain polysaccharide of the phenol-phase soluble lipopolysaccharide of Escherichia coli O:157:H7. Biochem. Cell Biol. 1986, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R.; Gerken, M.; Perry, M.B. Two-dimensional nuclear magnetic resonance at 500 MHz: the structural elucidation of a Salmonella serogroup N polysaccharide antigen. Can. J. Chem. 1986, 64, 255–264. [Google Scholar] [CrossRef]
- Vinogradov, E.; Conlan, J.W.; Perry, M.B. Serological cross-reaction between the lipopolysaccharide O-polysaccharide antigens of Escherichia coli O157:H7 and strains of Citrobacter freundii and Citrobacter sedlakii. FEMS Microbiol. Lett. 1998, 190, 157–161. [Google Scholar] [CrossRef]
- Senchenkova, S.N.; Shashkov, A.S.; Knirel, Y.A.; Wydra, K.; Witt, F.; Mavridis, A.; Rudolph, K. Structure of two O-polysaccharide of Xanthomonas cassavae GSPB 2437. Carbohydr. Res. 2004, 339, 157–160. [Google Scholar] [CrossRef]
- Leone, S.; Izzo, V.; Lanzetta, R.; Molinaro, A.; Parrilli, M.; Di Donato, A. The structure of the O-polysaccharide from Pseudomonas stutzeri OX1 containing two different 4-acylamido-4,6-dideoxy-residues, tomosamine and perosamine. Carbohydr. Res. 2005, 340, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Vinogradov, E.; Nomellini, J.F.; Smit, J. The core and O-polysaccharide structure of the Caulobacter crescentus lipopolysaccharide. Carbohydr. Res. 2015, 402, 111–117. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Vinogradov, E.; Altman, E. Structural studies of the core region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydr. Res. 2006, 341, 109–117. [Google Scholar] [CrossRef]
- Jimenez, N.; Canals, R.; Lacasta, A.; Kondakova, A.; Lindner, B.; Knirel, Y.A.; Merino, S.; Regue, M.; Tomas, J.M. Molecular analysis of three Aeromonas hydrophila AH-3 (Serotype O34) lipopolysaccharide core biosynthesis gene clusters. J. Bacteriol. 2008, 190, 3176–3184. [Google Scholar] [CrossRef]
- Merino, S.; Tomas, J.M. The Aeromonas salmonicida lipopolysaccharide core from different subspecies: the unusual subsp. pectinolytica. Front. Microbiol. 2016, 7, 125. [Google Scholar] [CrossRef]
- Russa, R.; Urbanik-Sypniewska, T.; Lindström, K.; Mayer, H. Chemical characterization of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch. Microbiol. 1995, 163, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Komaniecka, I.; Choma, A.; Lindner, B.; Holst, O. The structure of a novel lipid A from the lipopolysaccharide of Bradyrhizobium elkanii containing three mannose units in the backbone. Chem. Eur. J. 2010, 16, 2922–2929. [Google Scholar] [CrossRef]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Vilches, S.; Merino, S.; Tomas, J.M. Structure of the core region from the lipopolysaccharide of Plesiomonas shigelloides strain 302–73 (serotype O1). Eur. J. Org. Chem. 2009, 2009, 1365–1371. [Google Scholar] [CrossRef]
- Silipo, A.; Molinaro, A.; Sturiale, L.; Dow, J.M.; Erbs, G.; Lanzetta, R.; Newman, M.A.; Parrilli, M. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 2005, 280, 33660–33668. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]






| [M − H]‒ Observed | [M − H] ‒ Calculated | M Monoisotopic | Composition |
|---|---|---|---|
| 1570.012 | 1569.946 | 1570.953 | HexN2P2[14:0(3-OH)]2[i15:0(3-OH)](12:0)2 |
| 1698.172 | 1698.162 | 1699.169 | HexN2P1[14:0(3-OH)]3[i15:0(3-OH)](12:0)2 |
| 1768.187 | 1768.181 | 1769.188 | HexN2P2[14:0(3-OH)]4(12:0)2 |
| 1796.230 | 1796.139 | 1797.146 | HexN2P2[14:0(3-OH)]3[i15:0(3-OH)](12:0)2 |
| 1891.587 | 1891.598 | 1892.605 | [Hep6Hex1HexN2KdoanhP]-COO |
| 1935.611 | 1935.588 | 1936.595 | Hep6Hex1HexN2KdoanhP |
| 1977.615 | 1977.599 | 1978.606 | Hep6Hex1HexN2Ac1KdoanhP |
| 2555.127 | 2554.846 | 2555.853 | [6dHexNAc2HexN3Ac2Hep6Hex1KdoanhP]-H2O |
| 1664.534 | 1664.542 | 1665.550 | Hep5Hex2HexN1Kdoanh |
| 1694.541 | 1694.553 | 1695.560 | Hep6Hex1HexN1Kdoanh |
| 1856.592 | 1856.605 | 1857.613 | Hep6Hex2HexN1Kdoanh |
| Sugar Residue | Chemical Shifts (δ, ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 | ||
| →3)-α-l-Rhap4NAc-(1→ | A | 5.17 | 4.20 | 3.97 | 3.97 | 3.92 | 1.23 |
| 103.0 | 70.3 | 78.0 | 52.5 | 69.4 | 18.0 | ||
| →2)-α-l-Rhap4NAc-(1→ | B | 5.16 | 4.15 | 4.05 | 3.91 | 3.84 | 1.19 |
| 101.7 | 78.2 | 69.2 | 54.3 | 69.6 | 18.0 | ||
| →4)-α-d-GalpNAc-(1→ | C | 5.04 | 4.26 | 3.97 | 4.12 | 3.90 | 3.74, 3.81 |
| 95.0 | 50.9 | 69.2 | 77.2 | 72.7 | 62.2 | ||
| →3)-α-l-Rhap4NAc-(1→ | D | 5.00 | 3.91 | 3.94 | 3.98 | 3.92 | 1.23 |
| 103.0 | 67.0 | 74.3 | 52.3 | 69.4 | 18.0 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworaczek, K.; Kurzylewska, M.; Karaś, M.A.; Janczarek, M.; Pękala-Safińska, A.; Turska-Szewczuk, A. A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6. Mar. Drugs 2019, 17, 254. https://doi.org/10.3390/md17050254
Dworaczek K, Kurzylewska M, Karaś MA, Janczarek M, Pękala-Safińska A, Turska-Szewczuk A. A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6. Marine Drugs. 2019; 17(5):254. https://doi.org/10.3390/md17050254
Chicago/Turabian StyleDworaczek, Katarzyna, Maria Kurzylewska, Magdalena A. Karaś, Monika Janczarek, Agnieszka Pękala-Safińska, and Anna Turska-Szewczuk. 2019. "A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6" Marine Drugs 17, no. 5: 254. https://doi.org/10.3390/md17050254
APA StyleDworaczek, K., Kurzylewska, M., Karaś, M. A., Janczarek, M., Pękala-Safińska, A., & Turska-Szewczuk, A. (2019). A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6. Marine Drugs, 17(5), 254. https://doi.org/10.3390/md17050254