New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells
Abstract
:1. Introduction
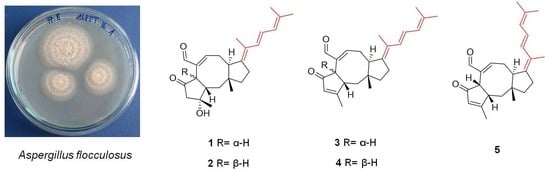
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Isolation of Compounds 1–9
3.4. Cytotoxicity Test by SRB Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Qadir, M.I.; Janbaz, K.H.; Ali, M. Novel drugs from marine microorganisms. Crit. Rev. Microbiol. 2011, 37, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug. Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs. 2016, 14, 19. [Google Scholar] [CrossRef]
- Nicoletti, R.; Vinale, F. Bioactive Compounds from Marine-Derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs. 2018, 16, 408. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.N.S.; Gubbala, S.P.; Chopra, P.; Upadhyayula, S.M.; et al. Aspergillus Secondary Metabolite Database, a resource to understand the Secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef] [PubMed]
- Trianto, A.; Widyaningsih, S.; Radjasa, O.K.; Pribadi, R. Symbiotic Fungus of Marine Sponge Axinella sp. Producing Antibacterial Agent. Environ. Earth. Sci. 2017, 55, 012005. [Google Scholar] [CrossRef]
- Wei, H.; Itoh, T.; Kinoshita, M.; Nakai, Y.; Kurotaki, M.; Kobayashi, M. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron 2004, 60, 6015–6019. [Google Scholar] [CrossRef]
- Au, T.K.; Chick, W.S.; Leung, P.C. The biology of ophiobolins. Life Sci. 2000, 67, 733–742. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.L.; William, A.T.; Wang, J.H.; Carl, L.T. Role of Calmodulin Inhibition in the Mode of Action of Ophiobolin A. Plant Physiol. 1985, 77, 303–308. [Google Scholar] [Green Version]
- Chai, H.; Yin, R.; Liu, Y.; Meng, H.; Zhou, X.; Zhou, G.; Bi, X.; Yang, X.; Zhu, T.; Zhu, W.; et al. Sesterterpene ophiobolin biosynthesis involving multiple gene clusters in Aspergillus ustus. Sci. Rep. 2016, 6, 27181. [Google Scholar] [CrossRef]
- Tian, W.; Deng, Z.; Hong, K. The Biological Activities of Sesterterpenoid-Type Ophiobolins. Mar. Drugs. 2017, 15, 7. [Google Scholar]
- Tsipouras, A.; Adefarati, A.A.; Tkacz, J.S.; Frazier, E.G.; Rohrer, S.P.; Birzin, E.; Rosegay, A.; Zink, D.L.; Goetz, M.A.; Singh, S.B.; et al. Ophiobolin M and analogues, noncompetitive inhibitors of ivermectin binding with nematocidal activity. Bioorg. Med. Chem. 1996, 4, 531–536. [Google Scholar] [CrossRef]
- Bladt, T.T.; Dürr, C.; Knudsen, P.B.; Kildgaard, S.; Frisvad, J.C.; Gotfredsen, C.H.; Seiffert, M.; Larsen, T.O. Bio-Activity and Dereplication-Based Discovery of Ophiobolins and Other Fungal Secondary Metabolites Targeting Leukemia Cells. Molecules 2013, 18, 14629–14650. [Google Scholar] [CrossRef] [Green Version]
- Nozoe, S.; Hirai, K.; Tsuda, K. The structure of zizannin-A and -B, C25-terpenoids isolated from helminthosporiumzizaniae. Tetrahedron Lett. 1966, 7, 2211–2216. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 a | 2 b | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| 1α | 1.63, m | 1.54, m | 1.22 (t, 13.2) | 1.44, m | 1.42, m |
| 1β | 1.84, m | 1.54, m | 2.15 (dd, 13.2, 3.6) | 2.13 (dd, 15.8, 4.5) | 2.13 (dd, 15.8, 4.5) |
| 2 | 2.19, m | 2.57, m | 2.72 (d, 12.9) | 3.32, overlap | 3.31, overlap |
| 4 | 2.47 (d, 16.6) | 2.57 (d, 18.9) | 6.01, s | 6.09, s | 6.08, s |
| 3.15 (d, 16.6) | 2.70 (d, 18.9) | ||||
| 6 | 3.33 (d, 10.5) | 3.47 (d, 11.8) | 3.53 (d, 3.6) | 4.29 (d, 7.2) | 4.28 (d, 7.2) |
| 8 | 6.84 (d, 6.7) | 7.44 (t, 8.6) | 6.91 (dd, 6.2, 2.3) | 7.24 (d, 6.7) | 7.21 (d, 6.8) |
| 9α | 2.94 (d, 20.8) | 2.48, m | 3.02 (d, 21.3) | 2.37, m | 2.30, m |
| 9β | 2.22, m | 2.48, m | 2.31, m | 2.37, m | 2.22, m |
| 10 | 3.19 (d, 13.0) | 2.28, m | 3.37 (d, 13.1) | 3.03 (d, 16.5) | 3.06 (d, 16.5) |
| 12α | 1.50, m | 1.43, m | 1.45, m | 1.46, m | 1.44, m |
| 12β | 1.63, m | 1.86, m | 1.65, m | 1.46, m | 1.44, m |
| 13α | 2.25 (dd, 14.8, 6.3) | 2.48, m | 2.31, m | 2.40, m | 2.37, m |
| 13β | 2.59, m | 2.95, m | 2.58 (dd, 14.8, 6.4) | 3.08, m | 3.03, m |
| 16 | 6.42 (d, 15.3) | 6.37 (d, 15.1) | 6.43 (d, 15.3) | 6.31 (d, 15.3) | 6.14 (d, 15.1) |
| 17 | 6.38 (dd, 15.3, 9.7) | 6.39 (dd, 15.1, 10.1) | 6.40(dd, 15.3, 9.3) | 6.34 (dd, 15.3, 10.3) | 6.31 (dd, 15.1, 10.5) |
| 18 | 5.93 (d, 9.7) | 5.92 (d, 10.1) | 5.90 (d, 9.3) | 5.88 (d, 10.3) | 5.94 (d, 10.5) |
| 20 | 1.47, s | 1.37, s | 2.12, s | 2.29, s | 2.27, s |
| 21 | 9.23, s | 9.28, s | 9.25, s | 9.46, s | 9.44, s |
| 22 | 0.94, s | 1.05, s | 0.99, s | 0.86, s | 0.84, s |
| 23 | 1.83, s | 1.96, s | 1.83, s | 1.72, s | 1.72, s |
| 24 | 1.81, s | 1.81, s | 1.78, s | 1.79, s | 1.77, s |
| 25 | 1.79, s | 1.81, s | 1.79, s | 1.80, s | 1.80, s |
| Position | 1 a | 2 b | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| 1 | 41.4 | 41.5 | 45.3 | 34.9 | 34.9 |
| 2 | 49.6 | 50.6 | 49.5 | 49.8 | 49.9 |
| 3 | 76.7 | 76.6 | 179.9 | 180.2 | 180.2 |
| 4 | 55.0 | 53.9 | 129.4 | 130.1 | 130.1 |
| 5 | 216.6 | 216.2 | 209.4 | 209.2 | 209.4 |
| 6 | 48.8 | 48.8 | 49.7 | 48.0 | 48.0 |
| 7 | 142.1 | 141.8 | 140.6 | 138.7 | 138.6 |
| 8 | 158.4 | 160.7 | 156.7 | 159.7 | 160.0 |
| 9 | 34.1 | 29.3 | 34.0 | 28.8 | 29.9 |
| 10 | 47.8 | 56.2 | 47.2 | 41.9 | 40.7 |
| 11 | 43.6 | 44.2 | 44.0 | 45.1 | 45.3 |
| 12 | 44.5 | 34.9 | 43.4 | 40.4 | 40.4 |
| 13 | 27.2 | 26.9 | 26.8 | 31.4 | 33.1 |
| 14 | 146.8 | 143.3 | 146.6 | 143.2 | 142.7 |
| 15 | 124.9 | 126.5 | 125.0 | 127.1 | 126.6 |
| 16 | 130.6 | 131.1 | 130.6 | 130.0 | 129.6 |
| 17 | 123.6 | 122.7 | 123.2 | 122.9 | 123.9 |
| 18 | 125.9 | 126.0 | 126.0 | 126.0 | 126.1 |
| 19 | 134.9 | 133.7 | 133.5 | 133.6 | 133.8 |
| 20 | 25.8 | 24.4 | 15.7 | 17.2 | 17.1 |
| 21 | 194.0 | 195.1 | 193.2 | 195.1 | 195.2 |
| 22 | 21.3 | 18.0 | 19.8 | 24.6 | 24.7 |
| 23 | 13.6 | 14.1 | 13.8 | 14.2 | 15.5 |
| 24 | 18.4 | 16.9 | 16.9 | 16.9 | 16.9 |
| 25 | 26.1 | 24.8 | 24.8 | 24.8 | 24.8 |
| Cell Lines a | GI50 (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ADR b | |
| HCT-15 | 0.21 | 0.44 | 0.96 | 1.24 | 1.67 | 0.24 | 0.21 | 0.30 | 0.22 | 0.13 |
| NUGC-3 | 0.19 | 0.50 | 0.88 | 1.07 | 1.53 | 0.22 | 0.20 | 0.22 | 0.20 | 0.15 |
| NCI-H23 | 0.18 | 0.61 | 1.40 | 1.50 | 1.84 | 0.24 | 0.16 | 0.22 | 0.22 | 0.15 |
| ACHN | 0.24 | 0.53 | 1.14 | 1.40 | 2.01 | 0.43 | 0.20 | 0.23 | 0.42 | 0.16 |
| PC-3 | 0.24 | 0.47 | 1.00 | 1.38 | 1.60 | 0.27 | 0.36 | 0.20 | 0.20 | 0.14 |
| MDA-MB-231 | 0.14 | 0.63 | 1.05 | 1.35 | 1.75 | 0.19 | 0.22 | 0.21 | 0.19 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-K.; Trinh, P.T.H.; Lee, H.-S.; Choi, B.-W.; Kang, J.S.; Ngoc, N.T.D.; Van, T.T.T.; Shin, H.J. New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Mar. Drugs 2019, 17, 346. https://doi.org/10.3390/md17060346
Choi B-K, Trinh PTH, Lee H-S, Choi B-W, Kang JS, Ngoc NTD, Van TTT, Shin HJ. New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Marine Drugs. 2019; 17(6):346. https://doi.org/10.3390/md17060346
Chicago/Turabian StyleChoi, Byeoung-Kyu, Phan Thi Hoai Trinh, Hwa-Sun Lee, Byeong-Woo Choi, Jong Soon Kang, Ngo Thi Duy Ngoc, Tran Thi Thanh Van, and Hee Jae Shin. 2019. "New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells" Marine Drugs 17, no. 6: 346. https://doi.org/10.3390/md17060346
APA StyleChoi, B. -K., Trinh, P. T. H., Lee, H. -S., Choi, B. -W., Kang, J. S., Ngoc, N. T. D., Van, T. T. T., & Shin, H. J. (2019). New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Marine Drugs, 17(6), 346. https://doi.org/10.3390/md17060346