Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides
Abstract
:1. Introduction
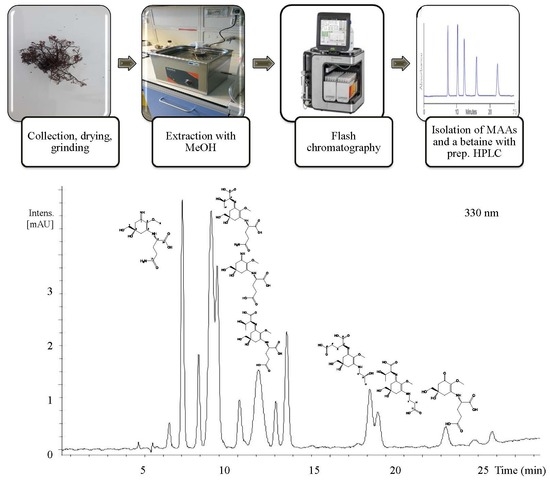
2. Results
2.1. Compound 1
2.2. Compound 2
2.3. Compound 3
2.4. Compound 4
2.5. Compound 5
2.6. Compound 6
2.7. Compound 7
2.8. Compound 8
3. Discussion and Conclusions
4. Materials and Methods
4.1. Biological Material
4.2. Instrumentation
4.3. Chemicals and Reagents
4.4. Extraction and Isolation
4.4.1. Bostrychine-A (1)
4.4.2. Bostrychine-B (2)
4.4.3. Bostrychine-C (3)
4.4.4. Bostrychine-D (4)
4.4.5. Bostrychine-E (5)
4.4.6. Bostrychine-F (6)
4.4.7. Mycosporine-Glutamic Acid (7)
4.4.8. Butyryl-lysine-betaine (8)
4.4.9. Choline (9)
4.5. Determination of the Absolute Configurations of Amino Acids in MAAs by the Advanced Marfey’s Method Using LC-MS
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AlgaeBase. Available online: http://www.algaebase.org/search/genus/detail/?genus_id=32785&-session=abv4:AC1F05FF0ef232A2D0Gl828F52DA (accessed on 26 February 2019).
- Zuccarello, G.C.; West, J.A. Multiple cryptic species: Molecular diversity and reproductive isolation in the Bostrychia radicans/B. moritziana complex (Rhodomelaceae, Rhodophyta) with focus on North American isolates. J. Phycol. 2003, 39, 948–959. [Google Scholar] [CrossRef]
- Muangmai, N.; Ammon, U.; Zuccarello, G. Cryptic species in sympatry: Nonrandom small-scale distribution patterns in Bostrychia intricata (Ceramiales, Rhodophyta). Phycologia 2016, 55, 424–430. [Google Scholar] [CrossRef]
- De Oliveira, A.L.L.; da Silva, D.B.; Turatti, I.C.C.; Yokoya, N.S.; Debonsi, H.M. Volatile constituents of Brazilian Bostrychia species (Rhodomelaceae) from mangrove and rocky shore. Biochem. Syst. Ecol. 2009, 37, 761–765. [Google Scholar] [CrossRef]
- Karsten, U.; Koch, S.; West, J.A.; Kirst, G.O. The intertidal red alga Bostrychia simpliciuscula Harvey ex J. Agardh from a mangrove swamp in Singapore: Acclimation to light and salinity. Aquat. Bot. 1994, 48, 313–323. [Google Scholar] [CrossRef]
- Martins, C.D.L.; Ramlov, F.; Nocchi Carneiro, N.P.; Gestinari, L.M.; Dos Santos, B.F.; Bento, L.M.; Lhullier, C.; Gouvea, L.; Bastos, E.; Horta, P.A.; et al. Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J. Appl. Phycol. 2013, 25, 1179–1187. [Google Scholar] [CrossRef]
- Felício, R.D.; Debonsi, H.M.; Yokoya, N.S. 4-(Hidroximetil)-Benzenossulfonato de potássio: Metabólito inédito isolado da alga marinha Bostrychia tenella (Rhodomelaceae, ceramiales). Quím. Nova 2008, 31, 837–839. [Google Scholar] [CrossRef]
- Oliveira, A.L.L.D.; Silva, D.B.D.; Lopes, N.P.; Debonsi, H.M.; Yokoya, N.S. Chemical constituents from red algae Bostrychia radicans (Rhodomelaceae): New amides and phenolic compounds. Quím. Nova 2012, 35, 2186–2188. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279. [Google Scholar]
- Karsten, U.; Sawall, T.; West, J.; Wiencke, C. Ultraviolet sunscreen compounds in epiphytic red algae from mangroves. Hydrobiologia 2000, 432, 159–171. [Google Scholar] [CrossRef]
- Kremer, B.P. 14C-Assimilate pattern and kinetics of photosynthetic 14CO2-assimilation of the marine red alga Bostrychia scorpioides. Planta 1976, 129, 63–67. [Google Scholar] [CrossRef]
- Karsten, U.; Kirst, G.O. Incomplete turgor pressure regulation in the “terrestial” red alga, Bostrychia scorpioides (Huds.) Mont. Plant Sci. 1989, 61, 29–36. [Google Scholar] [CrossRef]
- Huiskes, A.; Blom, C.W.P.M.; Rozema, J. Vegetation between land and sea: Structure and processes. In Plant Sciences, 3th ed.; Huiskes, A., Blom, C.W.P.M., Rozema, J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 70–71. [Google Scholar]
- Kirst, G.O. Osmotische Adaptation bei Algen. Naturwissenschaften 1985, 72, 125–132. [Google Scholar] [CrossRef]
- Reed, R.H.; Davison, I.R.; Chudek, J.A.; Foster, R. The osmotic role of mannitol in the Phaeophyta: An appraisal. Phycologia 1985, 24, 35–47. [Google Scholar] [CrossRef]
- Kremer, B.P. Distribution of alditols in the genus Bostrychia. Bioche. Syst. Ecol. 1976, 4, 139–141. [Google Scholar] [CrossRef]
- Karsten, U.; King, R.J.; Kirst, G.O. The distribution of d-sorbitol and d-dulcitol in the red algal genera Bostrychia and Stictosiphonia (Rhodomelaceae, Rhodophyta)―a re-evaluation. Br. Phycol. J. 1990, 25, 363–366. [Google Scholar] [CrossRef]
- Sánchez de Pedro, R. Ecophysiological study of the intertidal zonation of the estuarine rhodophytes Bostrychia scorpioides (Hudson) Montagne ex Kützing and Catenella caespitosa (Withering) L. M. Irvine. Doctoral Thesis, University of Malaga, Malaga, Spain, 21 December 2016. [Google Scholar]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharmacogn. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Lawrence, K.; Long, P.; Young, A. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef] [PubMed]
- La Barre, S.; Roullier, C.; Boustie, J. Mycosporine-Like Amino Acids (MAAs) in Biological Photosystems. In Outstanding Marine Molecules: Chemistry, Biology, Analysis, 3th ed.; La Barre, S., Kornprobst, J.M., Eds.; Wiley-Blackwell: Weinheim, Germany, 2014; pp. 333–360. [Google Scholar]
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240. [Google Scholar] [CrossRef]
- Vale, P. Can mycosporine-like amino acids act as multifunctional compounds in Gymnodinium catenatum (dinophyceae)? Photochem. Photobiol. 2016, 92, 264–275. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G.; Smith, B.E.; Irons, M.W.; Yang, M.H.; Roch, O.G.; Patel, A.V. Betaines and tertiary sulphonium compounds from 62 species of marine algae. Bioche. Syst. Ecol. 1992, 20, 373–388. [Google Scholar] [CrossRef]
- Hosseiniyan Khatibi, S.M.; Zununi Vahed, F.; Sharifi, S.; Ardalan, M.; Mohajel Shoja, M.; Zununi Vahed, S. Osmolytes resist against harsh osmolarity: Something old something new. Biochimie 2019, 158, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Stadmiller, S.S.; Pielak, G.J. Glycine betaine reverses osmotic shock induced protein destabilization in living cells. Biophys. J. 2017, 112, 57a. [Google Scholar] [CrossRef]
- Tiainen, M.; Maaheimo, H.; Niemitz, M.; Soininen, P.; Laatikainen, R. Spectral analysis of 1H coupled 13C spectra of the amino acids: Adaptive spectral library of amino acid 13C isotopomers and positional fractional 13C enrichments. Magn. Reson. Chem. 2008, 46, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Bernillon, J.; Bouillant, M.L.; Pittet, J.L.; Favre-Bonvin, J.; Arpin, N. Mycosporine glutamine and related mycosporines in the fungus Pyronema omphalodes. Phytochemistry 1984, 23, 1083–1087. [Google Scholar] [CrossRef]
- Snowden, M.K.; Baxter, J.H.; Mamula Bergana, M.; Reyzer, I.; Pound, V. Stability of N-acetylglutamine and glutamine in aqueous solution and in a liquid nutritional product by an improved HPLC method. J. Food Sci. 2002, 67, 384–389. [Google Scholar] [CrossRef]
- Uemura, D.; Katayama, C.; Wada, A.; Hirata, Y. Crystal and molecular structure of palythene possessing a novel 360 nm chromophore. Chem. Lett. 1980, 9, 755–756. [Google Scholar] [CrossRef]
- Furusaki, A.; Matsumoto, T.; Tsujino, I.; Sekikawa, I. Crystal and molecular structure of palythine trihydrate. Bull. Chem. Soc. Jpn. 1980, 53, 319–323. [Google Scholar] [CrossRef]
- Klisch, M.; Richter, P.; Puchta, R.; Häder, D.P.; Bauer, W. The stereostructure of Porphyra-334: An experimental and calculational NMR investigation. Evidence for an efficient ‘Proton Sponge’. Helv. Chim. Acta 2007, 90, 488–511. [Google Scholar] [CrossRef]
- White, J.D.; Cammack, J.H.; Sakuma, K.; Rewcastle, G.W.; Widener, R.K. Transformations of quinic acid. asymmetric synthesis and absolute configuration of Mycosporin I and Mycosporin-gly. J. Org. Chem. 1995, 60, 3600–3611. [Google Scholar] [CrossRef]
- White, J.D.; Cammack, J.H.; Sakuma, K. The synthesis and absolute configuration of mycosporins. A novel application of the Staudinger reaction. J. Am. Chem. Soc. 1989, 111, 8970–8972. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Bemis, J.E.; Bourne, D.J. Ultraviolet absorbing pigments from the marine sponge Dysidea herbacea: Isolation and structure of a new mycosporine. Comp. Bioch. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 115, 281–286. [Google Scholar] [CrossRef]
- Kamio, M.; Kicklighter, C.E.; Nguyen, L.; Germann, M.W.; Derby, C.D. Isolation and structural elucidation of novel mycosporine-like amino acids as alarm cues in the defensive ink secretion of the sea hare Aplysia californica. Helv. Chim. Acta 2011, 94, 1012–1018. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.; Middleton, C.; Plack, P.; Thomson, R. The isolation of four aminocyclohexenimines (mycosporines) and a structurally related derivative of cyclohexane-1:3-dione (Gadusol) from the brine shrimp, Artemia. Compa. Biochem. Physiol. Part B Biochem. Mol. Biol. 1985, 80, 755–759. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, U.; West, J. Ecophysiological studies on six species of the mangrove red algal genus Caloglossa. Funct. Plant Biol. 1993, 20, 729–739. [Google Scholar] [CrossRef]
- King, R.J.; Puttock, C. Morphology and taxonomy of Caloglossa (Delesseriaceae, Rhodophyta). Aust. Syst. Bot. 1994, 7, 89–94. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Traditional and medicinal use of Mangrove. In Mangroves and Salt Marshes, 1st ed.; Springer Verlag: Berlin, Germany, 1998; pp. 133–148. [Google Scholar]
- Karsten, U.; Lembcke, S.; Schumann, R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Rath, J.; Mandal, S.; Adhikary, S.P. Ecophysiology of the estuarine cyanobacterium Lyngbya aestuarii to varying salinity in vitro. Acta Physiol. Plant. 2014, 36, 409–419. [Google Scholar] [CrossRef]
- Kogej, T.; Gostincar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N. Mycosporines in extremophilic fungi-novel complementary osmolytes? Environ. Chem. 2006, 3, 105–110. [Google Scholar] [CrossRef]
- Karsten, U. Effects of salinity and ultraviolet radiation on the concentration of mycosporine-like amino acids in various isolates of the benthic cyanobacterium Microcoleus chthonoplastes. Phycol. Res. 2002, 50, 129–134. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R.; Tanaka, Y.; Takabe, T. Chapter 1 - osmoprotectant and sunscreen molecules from halophilic algae and cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–16. [Google Scholar]
- Hiscock, S. A Field Key to the British Red Seaweed, 1st ed.; Field Studies Council: Somerset, UK, 1986; pp. 44, 47. [Google Scholar]
- Fujii, K.; Ikai, Y.; Oka, H.; Suzuki, M.; Harada, K.I. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]



| Position | 1 (400 MHz) | 2 (600 MHz) | 3 (600 MHz) | 4 (600 MHz) | 5 (600 MHz) | 6 (600 MHz) |
|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 4 | 2.76, d (17.8) 2.83, d (17.8) | 2.77, d (17.4) 2.81, d (17.4) | 2.76, d (17.4) 2.81, d (17.4) | 2.80, d (18.0) 2.83, d (18.0) | 2.90, s | 2.95, s |
| 6 | 2.68, d (17.2) 2.97, d (17.2) | 2.75, d (17.4) 2.91, d (17.4) | 2.67, d (17.4) 2.95, d (17.4) | 2.77, d (17.4) 2.90, d (17.4) | 2.81, d (18.0) 2.76, d (18.0) | 2.74, d (17.4) 2.88, d (17.4) |
| 7 | 3.57, s | 3.57, s | 3.56, s | 3.56, s | 3.58, s | 3.59, s |
| 8 | 3.65, s | 3.70, s | 3.63, s | 3.69, s | 3.61, s | 3.63, s |
| 9 | 4.22, dd (8.0,4.8) | 4.23, dd (7.8/4.8) | 4.23, dd (8.4/4.8) | 4.43, dd (8.4/4.8) | 3.44, d (14.4/7.8) 3.51, d (14.4/2.4) | 3.76, t (6.0) |
| 10 | 4.02, m | 2.78, t (6.0) | ||||
| 11 | 2.18, m 2.26, m | 2.18, m 2.28, m | 2.12, m 2.24, m | 2.20, m 2.34, m | 1.22, d (6.0) | |
| 12 | 2.45, td (7.62.0) | 2.45, td (7.4/2.4) | 2.41, m | 2.57, td (7.2/1.8) | ||
| 1′ | 4.07, d (4.8) | 4.27, d (4.8) | 4.43, dd (7.8/5.4) | 4.35, m | ||
| 3′ | 4.31, m | 4.39, m | 2.19, m 2.34, m | 4.44, m | ||
| 4′ | 1.25, d (6.0) | 1.25, d (6.6) | 2.57, (t, 7.2) | 1.25, d (6.6) |
| Position | 1a (400 MHz) | 2 (600 MHz) | 3 (600 MHz) | 4 (600 MHz) | 5 (600 MHz) | 6 (600 MHz) | 8 (600 MHz) |
|---|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 1 | 163.6, C | 162.4, C | 163.6, C | 163.1, C | 161.8, C | 161.8, C | 174.8, CO |
| 2 | 127.7, C | 128.8, C | 127.8, C | 129.2, C | 128.8, C | 128.8, C | 81.8, CH |
| 3 | 163.9, C | 162.8, C | 164.4, C | 163.4, C | 164.5, C | 164.2, C | 28.7, CH2 |
| 4 | 36.4, CH2 | 36.2, CH2 | 36.5, CH2 | 36.1, CH2 | 36.1, CH2 | 35.8, CH2 | 25.5, CH2 |
| 5 | 74.2, C | 74.0, C | 74.1, C | 74.0, C | 74.0, C | 74.0, C | 30.9, CH2 |
| 6 | 38.5, CH2 | 36.0, CH2 | 38.5, CH2 | 36.2, CH2 | 36.0, CH2 | 36.1, CH2 | 41.6, CH2 |
| 7 | 70.2, CH2 | 70.3, CH2 | 70.3, CH2 | 70.4, CH2 | 70.4, CH2 | 70.4, CH2 | 180.0, CO |
| 8 | 61.9, CH3 | 62.4, CH3 | 62.0, CH3 | 62.5, CH3 | 62.2, CH3 | 62.3, CH3 | 40.6, CH2 |
| 9 | 61.4, CH | 61.5, CH | 61.7, CH | 60.2, CH | 53.1, CH2 | 42.3, CH2 | 22.0, CH2 |
| 10 | 179.1, CO | 179.3, CO | 179.3, CO | 178.1, CO | 69.5, CH | 36.7, CH2 | 15.6, CH3 |
| 11 | 30.5, CH2 | 30.6, CH2 | 30.8, CH2 | 29.7, CH2 | 22.3, CH3 | 178.2, CO | 54.6, CH3 |
| 12 | 34.2, CH2 | 34.3, CH2 | 35.4, CH2 | 33.2, CH2 | |||
| 13 | 181.1, CO | 181.2, CO | 182.6 CO | 180.3, CO | |||
| 1′ | 67.4, CH | 66.3, CH | 59.6, CH | 65.3, CH | |||
| 2′ | 178.1, CO | 176.9, CO | 178.0, CO | 176.5, CO | |||
| 3′ | 71.1, CH | 70.8, CH | 29.7, CH2 | 70.6, CH | |||
| 4′ | 22.3, CH3 | 22.2, CH3 | 33.2, CH2 | 22.1, CH3 | |||
| 5′ | 180.3, CO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Nguyen Ngoc, H.; Karsten, U.; Ganzera, M. Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. https://doi.org/10.3390/md17060356
Orfanoudaki M, Hartmann A, Miladinovic H, Nguyen Ngoc H, Karsten U, Ganzera M. Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Marine Drugs. 2019; 17(6):356. https://doi.org/10.3390/md17060356
Chicago/Turabian StyleOrfanoudaki, Maria, Anja Hartmann, Helena Miladinovic, Hieu Nguyen Ngoc, Ulf Karsten, and Markus Ganzera. 2019. "Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides" Marine Drugs 17, no. 6: 356. https://doi.org/10.3390/md17060356
APA StyleOrfanoudaki, M., Hartmann, A., Miladinovic, H., Nguyen Ngoc, H., Karsten, U., & Ganzera, M. (2019). Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Marine Drugs, 17(6), 356. https://doi.org/10.3390/md17060356