Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol
Abstract
:1. Introduction
2. Results and Discussion
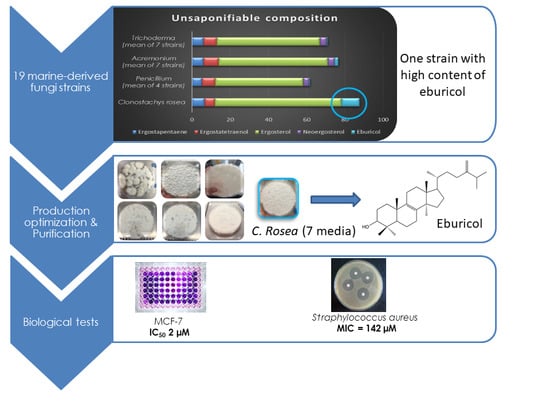
2.1. Marine Fungal Strains Screening
2.2. OSMAC Approach: Eburicol Production
2.3. Structural Identification of Eburicol
2.4. Biological Activities of Eburicol
2.4.1. Antiproliferative Activity of Eburicol against Human Cancer Cells
2.4.2. Antimicrobial Activity of Eburicol against Human Pathogenic Bacteria
3. Materials and Methods
3.1. Marine Fungal Strains
3.2. Culture Media Conditions
3.3. Lipid Extraction and Saponification
3.4. General Spectrometry Analysis
3.5. Production, Purification and Structural Elucidation of Sterol from C. rosea MMS 1090
3.6. Cell Viability Assay
3.7. Antibacterial Activity against Human Pathogens
3.8. Statistical analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Freter, C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, R.; Sackmann, E. Structure and Dynamics of Membranes: I. From Cells to Vesicles/II. Generic and Specific Interactions; Handbook of Biological Physics; Elsevier Science: Amsterdam, The Netherlands, 1995; ISBN 978-0-08-054191-4. [Google Scholar]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612. [Google Scholar] [CrossRef] [PubMed]
- Czub, J.; Baginski, M. Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol. Biophys. J. 2006, 90, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.S.; Cooke, D.T.; Carter, G.A. Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry 1989, 28, 1791–1804. [Google Scholar] [CrossRef]
- Kerr, R.G.; Kerr, S.L.; Pettit, G.R.; Herald, D.L.; Groy, T.L.; Djerassi, C. Sterols of marine invertebrates. 63. Isolation and structure elucidation of sutinasterol, the major sterol of the marine sponge Xestospongia sp. J. Org. Chem. 1991, 56, 58–62. [Google Scholar] [CrossRef]
- Nes, W.D.; Xu, S.; Haddon, W.F. Evidence for similarities and differences in the biosynthesis of fungal sterols. Steroids 1989, 53, 533–558. [Google Scholar] [CrossRef]
- Risley, J.M. Cholesterol biosynthesis: Lanosterol to cholesterol. J. Chem. Educ. 2002, 79, 377. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Mizuno, T.; Saito, H.; Nishitoba, T.; Kawagishi, H. Antitumor-active substances from mushrooms. Food Rev. Int. 1995, 11, 23–61. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, L.; Huang, J.; Li, W.; Pei, Y. Cytotoxic sterols from marine-derived fungus Penicillium sp. Nat. Prod. Res. 2006, 20, 381–384. [Google Scholar] [CrossRef]
- Chen, J.J.; Lin, W.J.; Liao, C.H.; Shieh, P.C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007, 70, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.; Monte, A. Antibacterial compounds from mushrooms II: Lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med. 2010, 76, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Leu, Y.L.; Chen, C.H.; Chao, C.H.; Shen, D.Y.; Chan, H.-H.; Lee, E.-J.; Wu, T.-S.; Wang, Y.-H.; Shen, Y.-C.; et al. Camphoratins A−J, potent cytotoxic and anti-inflammatory triterpenoids from the fruiting body of Taiwanofungus camphoratus. J. Nat. Prod. 2010, 73, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.S.; Chao, C.H.; Shen, D.Y.; Chan, H.H.; Chen, C.H.; Liao, Y.-R.; Wu, S.-J.; Leu, Y.-L.; Shen, Y.-C.; Kuo, Y.-H.; et al. Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorg. Med. Chem. 2011, 19, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.M.; Qi, F.M.; Li, J.; Jiang, C.X.; Hou, Y.; Shi, Y.P.; Di, D.L.; Zhang, J.W.; Wu, Q.X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef]
- Torres, S.; Cajas, D.; Palfner, G.; Astuya, A.; Aballay, A.; Pérez, C.; Hernández, V.; Becerra, J. Steroidal composition and cytotoxic activity from fruiting body of Cortinarius xiphidipus. Nat. Prod. Res. 2017, 31, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Kavita, K.; Singh, V.K.; Jha, B. 24-branched Δ5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Med. Extr. Microbiol. 2014, 169, 301–306. [Google Scholar] [CrossRef]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. (Tokyo) 2009, 62, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Genilloud, O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie Van Leeuwenhoek 2014, 106, 173–188. [Google Scholar] [CrossRef]
- Imhoff, F.J. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Wang, F.; Fang, Y.; Zhang, M.; Lin, A.; Zhu, T.; Gu, Q.; Zhu, W. Six new ergosterols from the marine-derived fungus Rhizopus sp. Steroids 2008, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.L.; Tao, M.H.; Dan, F.J.; Zhang, W.M. Two new sesquiterpenes from the marine fungus Eutypella scoparia FS26 from the South China Sea. Helv. Chim. Acta 2012, 95, 157–162. [Google Scholar] [CrossRef]
- Dias, C.A.; Ruiz, N.; Couzinet-Mossion, A.; Bertrand, S.; Duflos, M.; Pouchus, Y.F.; Barnathan, G.; Nazih, H.; Wielgosz-Collin, G. The marine-derived fungus Clonostachys rosea, source of a rare conjugated 4-Me-6E,8E-hexadecadienoic acid reducing viability of MCF-7 breast cancer cells and gene expression of lipogenic enzymes. Mar. Drugs 2015, 13, 4934–4938. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Srinivasan, R.A. Lipid and fatty acid composition of selected fungi grown on whey medium. J. Food Sci. 1984, 49, 950–951. [Google Scholar] [CrossRef]
- Brown, D.E.; Hasan, M.; Lepe-Casillas, M.; Thornton, A.J. Effect of temperature and pH on lipid accumulation by Trichoderma reesei. Appl. Microbiol. Biotechnol. 1990, 34, 335–339. [Google Scholar] [CrossRef]
- Suutari, M. Effect of growth temperature on lipid fatty acids of four fungi (Aspergillus niger, Neurospora crassa, Penicillium chrysogenum, and Trichoderma reesei). Arch. Microbiol. 1995, 164, 212–216. [Google Scholar] [CrossRef]
- Ruiz, N.; Dubois, N.; Wielgosz-Collin, G.; Robiou du Pont, T.; Bergé, J.P.; Pouchus, Y.F.; Barnathan, G. Lipid content and fatty acid composition of a marine-derived Trichoderma longibrachiatum strain cultured by agar surface and submerged fermentations. Process Biochem. 2007, 42, 676–680. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G.; Papanikolaou, S. Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar] [CrossRef]
- Dias, C.A.; Couzinet-Mossion, A.; Ruiz, N.; Le Bellec, M.; Gentil, E.; Wielgosz-Collin, G.; Bertrand, S. Sugar induced modification in glycolipid production in Acremonium sp. revealed by LC-MS lipidomic approach. Curr. Biotechnol. 2017, 6, 227–237. [Google Scholar]
- Barton, D.H.R.; Shioiri, T.; Widdowson, D.A. Biosynthesis of terpenes and steroids. Part V. The synthesis of ergosta-5,7,22,24(28)-tetraen-3β-ol, a biosynthetic precursor of ergosterol. J. Chem. Soc. C 1971, 1968–1974. [Google Scholar] [CrossRef]
- Ericsson, D.C.B.; Ivonne, J.N.R. Sterol composition of the macromycete fungus Laetiporus sulphureus. Chem. Nat. Compd. 2009, 45, 193–196. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Poyatos, J.A.; Jiménez, D.; Oliver, E. Phycomysterols and other sterols from the fungus Phycomyces blakesleeanus. J. Nat. Prod. 1998, 61, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Enrique Oltra, J.; Robinson, J.; Burke, P.V.; Jiménez, D.; Oliver, E. Sterols in erg mutants of Phycomyces: Metabolic pathways and physiological effects. Steroids 2002, 67, 403–409. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Lopez, J.F.; Grimalt, J.O.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 2008, 73, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore Nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Meeuwse, P.; Klok, A.J.; Haemers, S.; Tramper, J.; Rinzema, A. Growth and lipid production of Umbelopsis isabellina on a solid substrate—Mechanistic modeling and validation. Process Biochem. 2012, 47, 1228–1242. [Google Scholar] [CrossRef]
- Ratledge, C. Regulation of lipid accumulation in oleaginous microorganisms. Biochem. Soc. Trans. 2002, 30, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 2011, 102, 9737–9742. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Kelly, S.L. Molecular diversity of sterol 14α-demethylase substrates in plants, fungi and humans. FEBS Lett. 1998, 425, 263–265. [Google Scholar] [CrossRef]
- Bean, T.P.; Cools, H.J.; Lucas, J.A.; Hawkins, N.D.; Ward, J.L.; Shaw, M.W.; Fraaije, B.A. Sterol content analysis suggests altered eburicol 14α-demethylase (CYP51) activity in isolates of Mycosphaerella graminicola adapted to azole fungicides. FEMS Microbiol. Lett. 2009, 296, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.; Kelly, D.; Kelly, S. Molecular aspects of azole antifungal action and resistance. Drug Resist. Updat. 1999, 2, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Goad, J.L.; Akihisa, T. Analysis of Sterols; Blackie Academic & Professional (Chapman & Hall): London, UK, 1997; ISBN 978-94-010-7147-5. [Google Scholar]
- Shirane, N.; Murabayashi, A.; Masuko, M.; Uomori, A.; Yoshimura, Y.; Seo, S.; Uchida, K.; Takeda, K. Effect on ergosterol biosynthesis of a fungicide, SSF-109, in Botrytis cinerea. Phytochemistry 1990, 29, 2513–2520. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and -sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- De Almeida, T.L.; Monteiro, J.A.; Lopes, G.K.P.; Chiavelli, L.U.R.; Santin, S.M.D.O.; da Silva, C.C.; Kaplum, V.; Scariot, D.B.; Nakamura, C.V.; Ruiz, A.L.T.G.; et al. Chemical study and antiproliferative, trypanocidal and leishmanicidal activities of Maxillaria picta. Quím. Nova 2014, 37, 1151–1157. [Google Scholar]
- Akihisa, T.; Kithsiri Wijeratne, E.M.; Tokuda, H.; Enjo, F.; Toriumi, M.; Kimura, Y.; Koike, K.; Nikaido, T.; Tezuka, Y.; Nishino, H. Eupha-7,9(11),24-trien-3β-ol (“Antiquol C”) and other triterpenes from Euphorbia antiquorum latex and their inhibitory effects on Epstein−Barr virus activation. J. Nat. Prod. 2002, 65, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, E.S.; Amit, Z.; Swonger, M.M.; Kreishman, G.P.; Brooks, E.E.; Kreishman, M.; Jayasimhulu, K.; Parish, E.J.; Sun, H.; Kizito, S.A.; et al. Pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol], a rare sterol detected in the opportunistic pathogen Pneumocystis carinii hominis: Structural identity and chemical synthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 97–102. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H.N. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Aghaei, M.; Yazdiniapour, Z.; Ghanadian, M.; Zolfaghari, B.; Lanzotti, V.; Mirsafaee, V. Obtusifoliol related steroids from Euphorbia sogdiana with cell growth inhibitory activity and apoptotic effects on breast cancer cells (MCF-7 and MDA-MB231). Steroids 2016, 115, 90–97. [Google Scholar] [CrossRef]
- Dong, H.; Gou, Y.L.; Kini, R.M.; Xu, H.X.; Chen, S.X.; Teo, S.L.M.; But, P.P.H. A new cytotoxic polyhydroxysterol from soft soral Sarcophyton trocheliophorum. Chem. Pharm. Bull. (Tokyo) 2000, 48, 1087–1089. [Google Scholar] [CrossRef]
- Zovko Končić, M.; Ioannou, E.; Sawadogo, W.R.; Abdel-Razik, A.F.; Vagias, C.; Diederich, M.; Roussis, V. 4α-methylated steroids with cytotoxic activity from the soft coral Litophyton mollis. Steroids 2016, 115, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.H.; Firouzi, J.; Saeidnia, S.; Hajimehdipoor, H.; Jamili, S.; Rustaiyan, A.; Gohari, A.R. A cytotoxic hydroperoxy sterol from the brown alga, Nizamuddinia zanardinii. DARU J. Pharm. Sci. 2013, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, X.; Wang, J.; Liang, R.; Tian, Y.; Salendra, L.; Luo, X.; Zhou, X.; Yang, B.; Tu, Z.; et al. Three new highly oxygenated sterols and one new dihydroisocoumarin from the marine sponge-derived fungus Cladosporium sp. SCSIO41007. Steroids 2018, 129, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Gyosheva, M.; Tsvetkova, I.; Bankova, V. Antibacterial triterpenes from the threatened wood-decay fungus Fomitopsis rosea. Fitoterapia 2009, 80, 263–266. [Google Scholar] [CrossRef]
- Sharma, R.K.; Behari, M. Screening of the compound isolated from the leaves of Annona squamosa for antibacterial activity. Acta Cienc. Indica Chem. 1992, 18, 249–252. [Google Scholar]
- Niedermeyer, T.H.J.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Smania, A., Jr.; Monache, F.D.; Smania, E.D.F.A.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]




| Genus | Strain | TL Content (% w/DW) | Relative Unsaponifiable Composition (% of Total Unsaponifiable Fraction) as Free Compounds | ||||
|---|---|---|---|---|---|---|---|
| Ergostapentaene (6e) m/z 376 [M]+● | Ergostatetraenol (3f) m/z 394 [M]+● | Ergosterol (3e) m/z 396 [M]+● | Neoergosterol (4e) m/z 380 [M]+● | Eburicol (1b) m/z 440 [M]+● | |||
| Trichoderma | MMS13 | 13 | 4.8 | 4.5 | 77.2 | 2.4 | ND |
| MMS19 | 9 | 7.9 | 10.2 | 43.5 | 2.5 | Tr | |
| MMS510 | 5.8 | 4.3 | 4.8 | 66.8 | 3.1 | ND | |
| MMS639 | 12.9 | 5.1 | 5.5 | 63.8 | 3.5 | 0.7 | |
| MMS755 | 8.8 | 5.1 | 10.7 | 50.8 | 3.9 | 0.8 | |
| MMS852 | 17.3 | 6.5 | 3.1 | 79.2 | 4.3 | ND | |
| MMS1541 | 2.2 | 6.0 | 12.8 | 22.8 | 4.9 | 1.5 | |
| Mean | 10 ± 5 | 6 ± 1 | 7 ± 4 | 54 ± 20 | 3.5 ± 0.9 | 0.5 ± 0.4 | |
| Acremonium | MMS540 | 7.3 | 11.3 | 11.5 | 30.8 | 3.9 | ND |
| MMS594 | 18.1 | 5.4 | 9.3 | 44.7 | 3.5 | 2.1 | |
| MMS700 | 11.4 | 3.2 | 2.2 | 65.5 | 3.8 | 0.6 | |
| MMS713 | 9.2 | 3.3 | 6.5 | 66.3 | 3.5 | 0.5 | |
| MMS862 | 8 | 5.1 | 8.8 | 59.9 | 4.1 | 2.4 | |
| MMS887 | 20.1 | 7.4 | 8.9 | 56.1 | 3.2 | 5.4 | |
| MMS889 | 50.7 | 5.0 | 7.3 | 73.3 | 4.1 | ND | |
| Mean | 18 ± 15 | 6 ± 3 | 8 ± 3 | 57 ± 15 | 3.7 ± 0.3 | 2 ± 2 | |
| Penicillium | MMS42 | 5.7 | 4.0 | 5.5 | 60.3 | 3.1 | 0.6 |
| MMS330 | 2.9 | 6.1 | 7.6 | 49.3 | 3.4 | 0.7 | |
| MMS460 | 2.7 | 5.8 | 7.0 | 48.1 | 2.8 | 0.8 | |
| MMS646 | 33.5 | 5.1 | 8.3 | 26.5 | 2.8 | ND | |
| Mean | 11 ± 15 | 5.3 ± 0.9 | 7 ± 1 | 46 ± 14 | 3 ± 0.3 | 0.5 ± 0.1 | |
| Clonostachys | MMS1090 | 21.4 | 6.6 | 5.5 | 66.2 | ND | 9.4 |
| Culture Media | Unsaponifiable Content (% w/w of TL) | Relative Unsaponifiable Composition of MMS1090 Lipid Extract (% of Unsaponifiable Fraction) as Free Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ergostapentaene (6e) | 9-dehydroergosterol (5e) | Ergostatrienol (3b) | Ergosterol (3e) | Ergostadienol (2e) | Neoergosterol (4e) | Fungisterol (2d) | Lanosterol (1a) | Eburicol (1b) | Others | ||
| DCA | 8.8 ± 1.4 | 5.9 ± 0.6 | 5.0 ± 0.5 | 1.9 ± 0.2 | 63.2 ± 3.3 | 4.7 ± 0.6 | 2.2 ± 0.2 | 0.8 ± 0.2 | Tr (1) | 8.0 ± 1.3 | 8.2 ± 1.1 |
| GCA | 9.5 ± 1.1 | 4.7 ± 0.9 | 5.3 ± 1.1 | 3.1 ± 0.4 | 68.2 ± 6.3 | 2.8 ± 0.3 | 2.5 ± 0.4 | Tr (1) | 0.3 ± 0.2 | 2.7 ± 0.8 | 13.7 ± 2.0 |
| YES | 12.5 ± 2.1 | 3.8 ± 0.5 | 3.1 ± 0.7 | 1.1 ± 0.1 | 61.8 ± 3.4 | 4.2 ± 0.5 | 2.1 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.3 | 11.7 ± 2.4 | 10.6 ± 1.2 |
| CYA | 10.6 ± 2.4 | 5.1 ± 1.2 | 5.5 ± 0.8 | 1.4 ± 0.5 | 65.1 ± 3.8 | 8.9 ± 1.3 | 1.8 ± 0.5 | 1.7 ± 0.1 | ND (1) | 5.6 ± 0.5 | 4.9 ± 1.2 |
| KMS | 7.5 ± 3.5 | 4.7 ± 0.9 | 5.9 ± 0.7 | 2.8 ± 0.1 | 61.5 ± 3.8 | 3.6 ± 0.8 | 2.6 ± 0.7 | 1.3 ± 1.1 | Tr (1) | 3.81 ± 0.06 | 12.7 ± 1.2 |
| PDA | 7.5 ± 1.8 | 3.3 ± 0.5 | 1.7 ± 0.3 | 0.9 ± 0.1 | 80.9 ± 1.3 | 5.4 ± 0.5 | 1.97 ± 0.02 | 0.6 ± 0.3 | ND (1) | 2.3 ± 0.6 | 2.9 ± 0.4 |
| MEA | 10.0 ± 2.5 | 3.4 ± 0.3 | 3.6 ± 0.4 | 1.3 ± 0.2 | 72.4 ± 4.1 | 2.9 ± 0.4 | 2.0 ± 0.5 | 0.8 ± 0 | Tr (1) | 7.5 ± 0.8 | 6.1 ± 1.3 |
| Position | 1H NMR δ (ppm) | m, J (Hz) | 13C NMR δ (ppm) |
|---|---|---|---|
| 1 | 1.35–2.34 | M | 35.6 |
| 2 | 1.35–2.34 | M | 27.8 |
| 3 | 3.24 | dd, 4.7 and 11.5 | 79.0 |
| 4 | - | - | 38.6 |
| 5 | 1.35–2.34 | M | 50.4 |
| 6 | 1.35–2.34 | M | 18.2 |
| 7 | 1.35–2.34 | M | 28.2 |
| 8 and 9 | - | - | 134.4 |
| 10 | - | - | 37.0 |
| 11 | 1.35–2.34 | M | 21.0 |
| 12 | 1.35–2.34 | M | 26.5 |
| 13 | - | - | 44.5 |
| 14 | - | - | 49.8 |
| 15 | 1.35–2.34 | M | 31.3 |
| 16 | 1.35–2.34 | M | 31.0 |
| 17 | 1.35–2.34 | M | 50.4 |
| 18 | 0.70 | S | 15.7 |
| 19 | 0.99 | S | 18.7 |
| 20 | 1.35–2.34 | M | 36.5 |
| 21 | 0.93 | d, 6,4 | 19.1 |
| 22 | 1.35–2.34 | M | 35.0 |
| 23 | 1.35–2.34 | M | 30.8 |
| 24 | - | - | 156.9 |
| 25 | 2.24 | M | 22.0 |
| 26 | 1.04 | d, 6.9 | 33.8 |
| 27 | 1.03 | d, 6.9 | 21.9 |
| 28 | 4.67 and 4.72 | d and s, 1.2 | 105.9 |
| 29 | 0.82 | S | 15.4 |
| 30 | 1.01 | S | 27.9 |
| 31 | 0.89 | S | 24.3 |
| OH | 5.35 | m |
| Sterol Compounds | IC50 (µM) | Reference | |||
|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | NSCLC-N6-L16 | A549 | ||
| Eburicol | 2.0 ± 0.7 | 15.7 ± 0.9 | 24.5 ± 0.5 | 38 ± 3 | Present study |
| Obtusifoliol | 29.3 | 41.8 | - | - | [51] |
| Gorgosterol | 71.2 | - | - | - | [52] |
| 4-methyl sterol | - | - | - | 20.4 | [53] |
| Ketone sterols | - | - | - | 4.9 to >100 | [22] |
| Hydroperoxysterol | 20.5 | - | - | 40.4 | [54] |
| Polyhydroxysterols | 10.6–71.9 | - | - | - | [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos Dias, A.C.; Couzinet-Mossion, A.; Ruiz, N.; Lakhdar, F.; Etahiri, S.; Bertrand, S.; Ory, L.; Roussakis, C.; Pouchus, Y.F.; Nazih, E.-H.; et al. Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol. Mar. Drugs 2019, 17, 372. https://doi.org/10.3390/md17060372
Dos Santos Dias AC, Couzinet-Mossion A, Ruiz N, Lakhdar F, Etahiri S, Bertrand S, Ory L, Roussakis C, Pouchus YF, Nazih E-H, et al. Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol. Marine Drugs. 2019; 17(6):372. https://doi.org/10.3390/md17060372
Chicago/Turabian StyleDos Santos Dias, Ana Camila, Aurélie Couzinet-Mossion, Nicolas Ruiz, Fatima Lakhdar, Samira Etahiri, Samuel Bertrand, Lucie Ory, Christos Roussakis, Yves François Pouchus, El-Hassane Nazih, and et al. 2019. "Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol" Marine Drugs 17, no. 6: 372. https://doi.org/10.3390/md17060372
APA StyleDos Santos Dias, A. C., Couzinet-Mossion, A., Ruiz, N., Lakhdar, F., Etahiri, S., Bertrand, S., Ory, L., Roussakis, C., Pouchus, Y. F., Nazih, E. -H., & Wielgosz-Collin, G. (2019). Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol. Marine Drugs, 17(6), 372. https://doi.org/10.3390/md17060372